-
Host Cell Protein Detection Kits
- CHO Host Cell Protein ELISA Kit
- E. coli Host Cell Protein ELISA Kit
- HEK 293 Host Cell Protein ELISA Kit
- Pichia pastoris Host Cell Protein ELISA Kit
- Ogataea polymorpha Host Cell Protein ELISA Kit, G3
- Saccharomyces cerevisiae Host Cell Protein ELISA Kit, G3
- Spodoptera fugiperda 9 (Sf9) Host Cell Protein ELISA Kit, G3 (Applicable to Sf9 and related cell lines)
- Dilution Buffer
-
Bioprocess lmpurity ELISA Kits
- Human Immunoglobulin G (IgG) ELISA Kit
- Goat Immunoglobulin G (IgG) ELISA Kit
- Human Serum Albumin (HSA) ELISA Kit
- NE01I0004 Human Insulin (INS) ELISA Kit ( Double Antibody Sandwich Method )
- Bovine Serum Albumin (BSA) ELISA Kit
- Dextran Sulfate Salt Detection Kit (Spectrophotometric Method-200 Tests)
- Protein L (PL) ELISA Kit
- Kanamycin (KA) ELISA Kit
- Human Immunoglobulin A (IgA) ELISA Kit
- Human Immunoglobulin M (IgM) ELISA Kit
- Mouse Immunoglobulin G (IgG) ELISA kit
- Bovine Immunoglobulin G (IgG) ELISA kit
- Protein A (PA) ELISA kit-Boiling
- Protein A (PA) ELISA Kit
- Diluent Buffer for Protein L ELISA kit
-
Host Cell DNA Residue Detection Kits
- CHO HCD Residue Detection Kits
- NS0 HCD Residue Detection Kits
- Vero HCD Residue Detection Kits
- E.coli HCD Residue Detection Kits
- HEK293 HCD Residue Detection Kits
- PP HCD Residue Detection Kits
- MDCK Host Cell DNA Residue Detection Kit
- Saccharomyces Cerevisiae Host Cell DNA Residue Detection Kit
- Saccharomyces Cerevisiae Host Cell DNA Residue Detection Kit_copy20260304112530
- Ogataea polymorpha Host Cell DNA Residue Detection Kit
- Magnetic Residual DNA Sample Preparation Kit
- DNA Dilution Buffer
- Residual Total RNA Detection Kits (qRT-PCR)
- Antibodies
- Recombinant Proteins
- ELISA Kits
- Cellular Component Protein Library
- Plasmids
- Promotions
-
BSA: Familiar Yet Unfamiliar – Launch of the Anti-Interference BSA ELISA Kit
Bovine Serum Albumin (BSA) is widely used in in vitro diagnostics and the industrial production of biopharmaceuticals, making it a familiar name in the field. However, its source, structure, function,...
Feb.24, 2026Read More > -
HEK293 HCP ELISA: Fit for Viral Purification Processes, That’s What Truly Matters
Origin of HEK 293Human Embryonic Kidney 293 cells, commonly referred to as HEK 293, HEK-293, or 293 cells, are an immortalized cell line isolated from the embryo of a pregnant female in the 1970s[1].I...
Feb.11, 2026Read More > -
Yeast Family: Pichia pastoris (GS115) HCP ELISA
1. Application Background:Pichia pastoris is one of the most widely used expression systems for innovative antibody expression in vaccines nowadays. Yeast is extensively applied in modern industry [1]...
Jan.20, 2026Read More >
Company Profile
Shanghai BlueGene Biotech CO., LTD.
BlueGene Biotech, established in June 2008, is a high-tech company focusing on the field of life science and biotechnology, which integrates product development and research, production and sales in one to provide a wide range of products and scientific and technical services worldwide to customers. BlueGene Biotech is mainly dedicated to researching life science, including molecular biology, cell biology, immunology, and other application fields.
With the continued development, BlueGene Biotech has set its R&D team in the Medical Valley of Zhangjiang International Medical Zone (located at Building 6, Lane 889, Ziping Road, Pudong New Area, Shanghai, China), which is the most concentrated and powerful biomedical R&D base in Shanghai, China. To become a highly competitive and professional scientific research company, BlueGene Biotech has continuously delivered the highest quality biological reagents to global customers for more than 10 years.
BlueGene Biotech always believe that the best research and development not only comes from good products, good business and good profits but also from good customer satisfaction. We are always determined to set on long-term development.

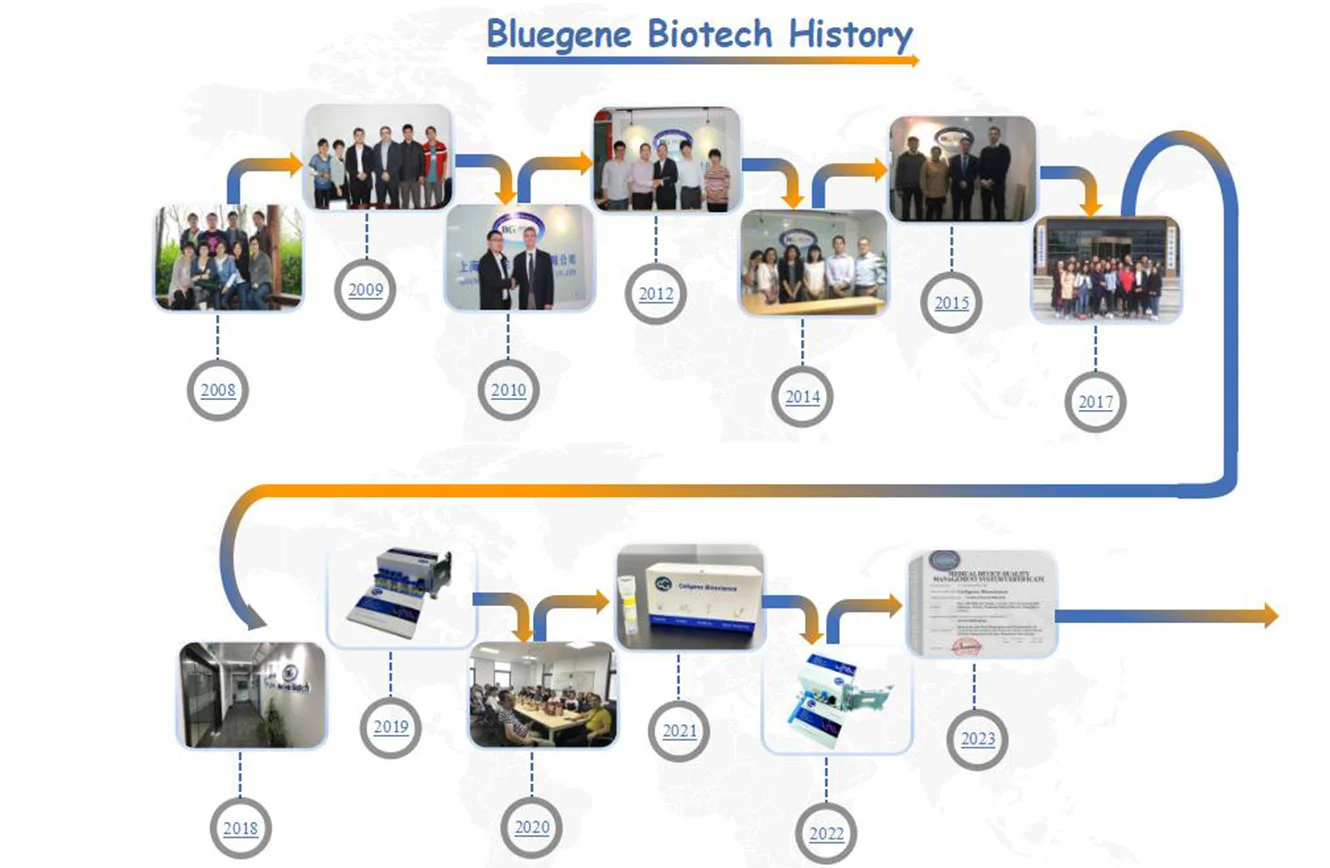
Company History
In 2025, Cellgene Bioscience Co., Ltd. attained another notable achievement. In the Laboratory Proficiency Testing Program for Residual Vero Cell Protein Quantification in Vaccines (Program No.: NIFDC-PT-526), organized and implemented by the National Institutes for Food and Drug Control (NIFDC), the test results submitted by Cellgene Bioscience were officially rated as "satisfactory", and the company was awarded an authoritative certificate issued by NIFDC.

On August 1st, 2024, Shanghai Bluegene Biotech/Cellgene Bioscience has relocated to Building 6, Zhangjiang Gene Island. This move marks a significant milestone in our corporate development. From this new starting point, we remain committed to advancing our core products, such as drug residue detection kits, and will increase investment in both R&D and market expansion. We look forward to collaborating with our clients and partners to create a shared future.

In 2023, through the great efforts of all staff, Cellgene Bioscience obtained the certificate of IS013485 for Research and Development and Production of Cytokine Recombinant Protein, Host Cell Protein ELISA Detection Kit (for Research Use Only).

In 2022, BlueGene Biotech has started to design and upgrade the company's new website. Through the great efforts of our R&D team, a new product CHO Host Cell Protein ELISA Kit has been launched.

In 2021, through the great efforts of our R&D team, more than 12 new recombinant proteins had been launched to market. These proteins are applied for antibody preparation, immunoassay, subcellular localization, and interacting protein identification.

In 2019, the R&D department successfully finished debugging some new sandwich ELISA products for putting official production soon. We have set up a new company named Cellgene Bioscience.
In 2018, the 700m2 GMP Workshop was completed the decoration work as planned, located at Shanghai International Medical Park in Shanghai Pudong district. And a lot of lab equipment was introduced to further expand and assist our research in many lab projects, and develop new products. After that, a series of ELISA kits for the diagnosis of animal diseases was launched.

In 2017, BlueGene Biotech R&D center (research and development center) moved to Zhangjiang Pharm Valley, BlueGene Biotech International Business Center moved to Pudong Zhangjiang New District in Zhangjiang High-tech Park, which is adjacent to BlueGene new lab located in Zhangjiang Pharma Valley-National High-tech innovation Service Center.

In 2016, BlueGene Biotech introduced Lab equipment to further expand the laboratory, we upgraded the ELISA kits product line, and tens of thousands of new products were added to the catalog. We were honored to have NPO Immunotex as our partner in Russia.

In 2015, we started the cooperation with 2B Scientific Ltd, ARP American Research Products, Inc. In the same year, BlueGene Biotech technology department developed a variety of new ELISA kits with higher sensitivity, which were validated by a third-party company.

In 2014, we were honored to have BioMart Co., Hongjing, Bio-connect, and BARIA s.r.o. as our partners.

In 2012, BlueGene Biotech expanded the technical team to enrich the company development and management on a new scale, we started cooperation with Japan Osaka Pharmaceutical Research Company, Genxbio Health Sciences Pvt. Ltd., DELTACLON S.L.

In 2011, as the market continues to expand, BlueGene Biotech achieved all-around development in product scale and brand establishment then moved to a new and large office building in Shanghai Putuo District. In the same year, we started cooperation with a Dubai customer from the United Arab Emirates, and after, antibodies-online, KAMIYA, etc.

In 2010, BlueGene Biotech build cooperation with Alibaba International Station to expand the sales market,, the sales market was rapidly developed in the Americas, Europe, and Asia to establish international distributor channels.

In 2009, the R&D center was established and settled down, and one-step method cytokine Elisa kits were successfully developed. And the company trademark BlueGene Biotech was registered.

In 2008, Shanghai BlueGene Biotech CO., Ltd was established in China of Shanghai and built an outstanding and professional team to provide technical support for product research and development. Soon after, the company website was revised and launched.


