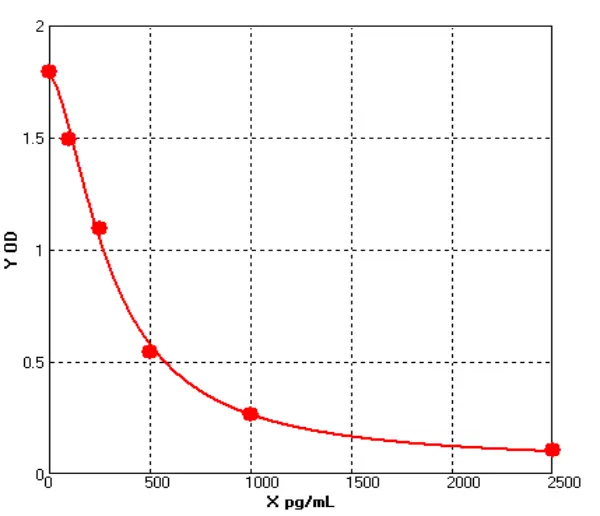
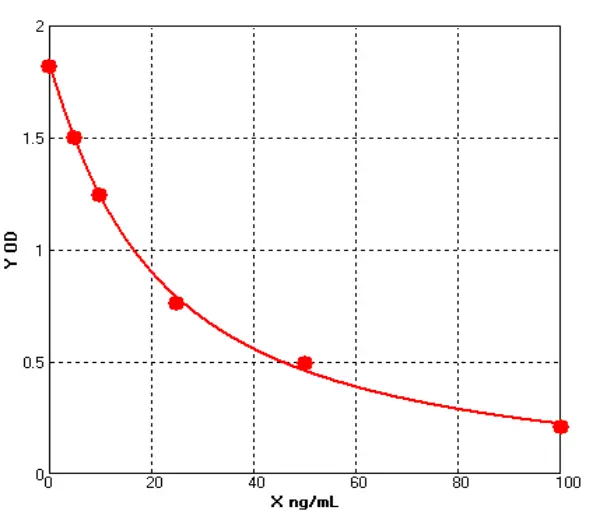
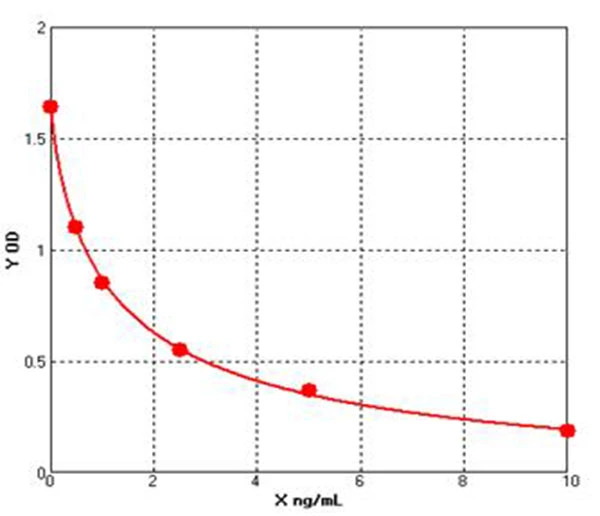
How Are IgG Levels Measured?
ELISA (Enzyme-linked immunosorbent assay) is a commonly used method for measuring IgG (Immunoglobulin G) levels in clinical and research applications. In the case of human IgG ELISA kits, these assays are designed to measure IgG levels in human samples such as serum, plasma, and other biological fluids. In this article, we will explore the process of measuring IgG levels using ELISA and the factors that can influence the accuracy and reliability of the assay.
ELISA for measuring human IgG levels
ELISA is a powerful technique for measuring the presence and concentration of a specific protein in a sample. The basic principle of the ELISA involves immobilizing the antigen (in this case, human IgG) onto a solid surface, such as a microtiter plate, and then using specific antibodies to detect and quantify the antigen. The assay can be performed in a variety of formats, including direct, indirect, sandwich, and competitive ELISAs, depending on the specific application and goals of the assay.
In the case of human IgG ELISA kit, the assay typically involves coating a microtiter plate with a known concentration of purified human IgG. After washing away any unbound proteins, the sample (such as serum or plasma) is added to the plate, and any IgG in the sample will bind to the coated IgG on the plate. Next, a secondary antibody that is specific for human IgG is added to the plate, followed by an enzyme-linked tertiary antibody that will produce a signal when it binds to the secondary antibody. The amount of signal produced is proportional to the amount of human IgG in the sample, and can be measured using a spectrophotometer or other detection system.
Factors that can affect the accuracy of human IgG ELISA kits
While custom elisa kits is a powerful and widely used technique, there are several factors that can affect the accuracy and reliability of the assay. These factors include:
Specificity and sensitivity of the antibodies: The antibodies used in the ELISA must be highly specific for human IgG, and should not cross-react with other proteins or immunoglobulins present in the sample. The antibodies must also be sensitive enough to detect low levels of IgG in the sample.
Sample matrix: The sample matrix can have a significant impact on the accuracy of the ELISA. For example, some sample matrices (such as serum or plasma) may contain interfering substances that can affect the binding of the antibodies to the IgG on the plate, leading to false positive or false negative results.
Standardization: The ELISA must be standardized to ensure consistent and reliable results. This includes using appropriate controls, calibrators, and reference materials, as well as carefully controlling the conditions of the assay, such as temperature, pH, and incubation times.
Operator variability: The ELISA is a complex assay that requires careful attention to detail and consistent performance to ensure accurate results. Variability in the performance of the assay by different operators can lead to inconsistencies in the results.
Human IgG1 ELISA kit is a powerful tool for measuring the presence and concentration of IgG in human samples, and can be used in a variety of clinical and research applications. However, as with any assay, there are several factors that can affect the accuracy and reliability of the results. By carefully controlling these factors, and by using high-quality reagents and equipment, researchers and clinicians can obtain accurate and reliable measurements of human IgG levels, enabling them to better understand the immune system and diagnose and treat diseases.