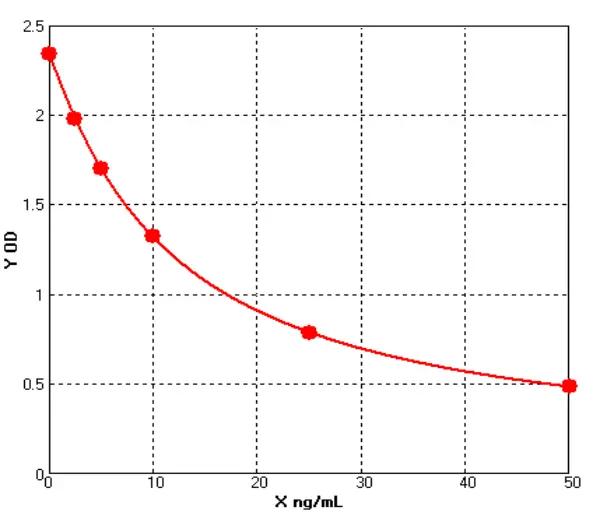
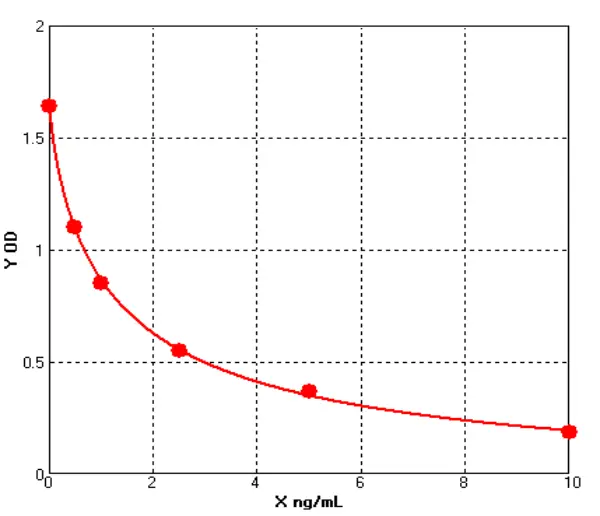
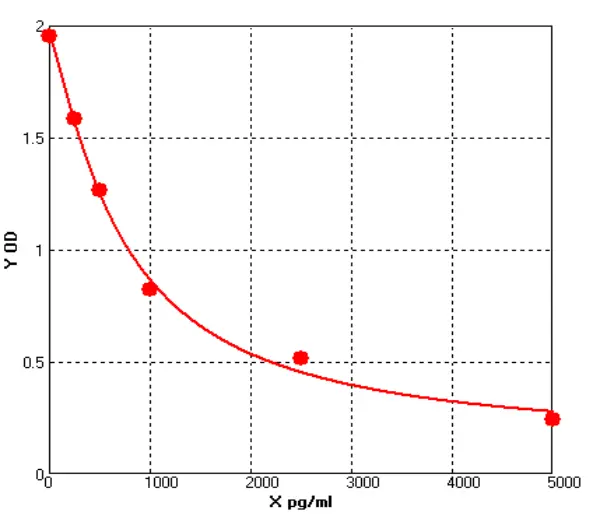
Principle of His-tag Protein Purification
His-tag in the His-tag protein purification kit can interact specifically with a variety of metal ions, such as Ca2+, Mg2+, Ni2+, Cu2+, Fe3+, etc. The principle is to use the properties of the protein surface so that it is adsorbed on the gel column, thus achieving the purpose of separating and purifying the protein. Histidine (His) residues with an imidazole group can form coordination bonds with Ni2+, Co2+, and other transition metal ions and selectively bind to the metal ions, which can be immobilized on the chromatography medium with chelating ligands, so proteins with His-Tag can selectively bind to the medium when passing through the chromatography medium assembled with metal ions, while other impurities The protein is not bound or only weakly bound. The his-tag recombinant protein bound to the medium can be competitively eluted by increasing the concentration of imidazole in the buffer, resulting in a higher purity His-Tag protein. The most widely used in affinity purification experiments is Ni2+. Depending on the binding group, Ni2+ affinity chromatography columns can be divided into two categories: Ni-IDA and Ni-NTA. Ni2+ has six chelating valencies, with Ni-IDA chelating the trivalent and Ni-NTA the tetravalent. However, its weak binding force makes it easy for metal ions to leach out during the elution phase and bind tightly to the target protein, resulting in low yields of the isolated protein, impure products, metal ion contamination, etc. The particle size of NTA is uniform, smaller and Chelated nickel is more stable and can tolerate higher reducing agents, making the packing more stable and the nickel ions less likely to be dislodged, hence the choice of Ni-NTA affinity chromatography columns for protein purification.