Detection And Quality Control Of Host Proteins
1. HCP Definition
Host proteins (HCP) form a major part of the process-related impurities in the production of biologics. The amount of residual HCP in a pharmaceutical product is often considered a critical quality attribute (CQA) because of its potential to affect product safety and efficacy. Therefore, the regulatory requirement is to monitor the removal of HCP from biologics during bioprocess development.
HCPs are proteins produced or encoded by host cells for the production of recombinant therapeutic proteins. Recombinant therapeutic proteins are typically produced from genetically modified prokaryotic or eukaryotic host cells using cell culture or fermentation techniques. Genetic engineering allows the host cells to be transformed to selectively express the target protein. During recombinant protein production, host cells also co-produce proteins associated with normal cellular functions, such as cell growth, proliferation, survival, gene transcription, and protein synthesis. Other non-essential proteins can also be released into the cell culture medium as a result of apoptosis, death, and lysis.
2. Detection And Monitoring Of Residual HCP
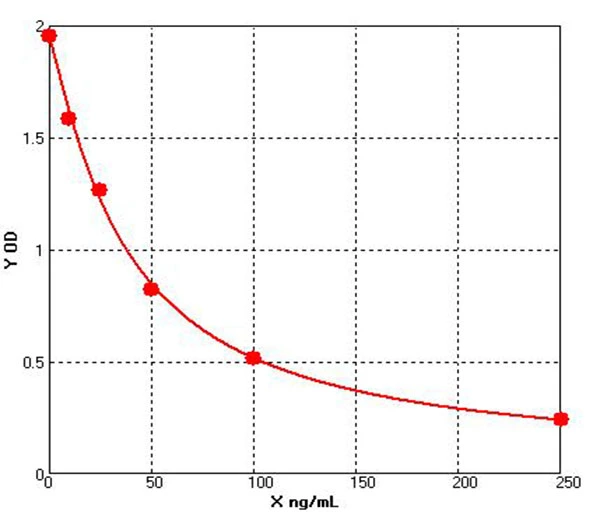
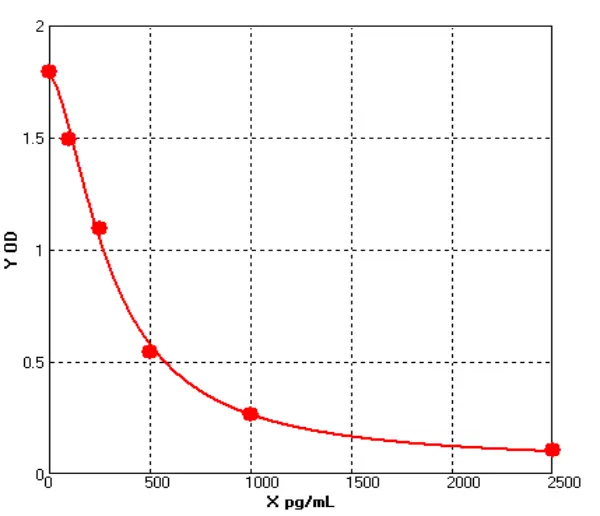
To date, immunoassays, usually in the form of sandwich enzyme-linked immunosorbent assays (ELISA), remain the industry gold standard for HCP measurement due to their high sensitivity and throughput. the composition and abundance of HCP are unique to their respective hosts and the manufacturing processes used for biologics production. At the same time, different host cells and manufacturing processes may produce similar amounts of some HCP. the amount of host cell-derived protein varies from host to host. For example, Escherichia coli (E. coli) has ~4300 genes, while Chinese hamster ovary (CHO HCP) cells have ~30,000 genes. Although not every host gene is transcribed and translated into a protein, the complexity of the host genome and the post-translational modifications present in mammalian cells make it almost impossible to know the complete HCP composition for a given production process. As this HCP is potentially immunogenic, the generally accepted method of assessing the presence of HCP is by immunoassay. Theoretically, injection of an HCP mixture into an animal (e.g. rabbit, goat, or chicken) will trigger an immune response and the animal will produce anti-HCP antibodies against these exogenous proteins. Although the major components of all HCP are not known, polyclonal antibodies produced in animal cells should be able to recognize the content of most, if not all, of the proteins in the HCP mixture. Using these polyclonal antibodies, a multi-analyte sandwich ELISA can be developed.
A universal HCP ELISA developed using polyclonal antibodies produced against parental cell lysates or cell culture supernatants can detect most HCP species but may not detect a subset of proteins specific to a particular manufacturing process. The Universal ELISA kit is commercially available and has the advantage of eliminating lengthy assay development times and only requires following the steps indicated in yellow before being used for process development. This makes the HCP elisa kit ideal for early development. At a later stage (clinical phase III or commercial production stage), a cell line-specific platform assay or upstream process-specific ELISA assay is often required to mitigate the risks associated with more generic commercial assays. Typically, platform- or process-specific HCPs are generated by a similar upstream process under empty cell cultures (mimics) without upstream products encoded to represent HCPs in platform-producing cultures. PAb generated by immunization of animals with these HCPs, after overlay assessment, will be used for ELISA development. Polyclonal antibodies should recognize most of the HCP co-produced with the drug. after identification and validation, the HCP ELISA can be used as a QC release assay for the stock solution.