Human Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) Protein, Recombinant
Introduction:
Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), also known as Colony Stimulating Factor 2 (CSF2), is a monomeric glycoprotein. Unlike Granulocyte Colony Stimulating Factor (G-CSF), which specifically promotes the proliferation and maturation of neutrophils, GM-CSF affects a broader range of cell types, particularly macrophages and eosinophils.
High levels of GM-CSF have been detected in the joints of patients with rheumatoid arthritis, and targeting GM-CSF as a biological target can reduce inflammation or tissue damage. In critically ill patients, GM-CSF has been trialed as an immunosuppressive therapy, showing potential in restoring the function of monocytes and neutrophils.
The functions of GM-CSF include: Hematopoiesis and differentiation of bone marrow lineage cells; Development and maintenance of alveolar macrophages; Recruitment and differentiation of monocyte-derived dendritic cells (DCs), including the production of IL-23 and polarization of TH17 T cells; Maturation and antigen presentation by conventional DCs, such as CD103-expressing DCs in the skin and small intestine; Polarization of M1 macrophages, including the production of pro-inflammatory cytokines, phagocytosis, and antigen presentation; Priming and activation of neutrophils, including processes such as phagocytosis, oxidative bursts, and nitric oxide generation.
Activity assay protocol
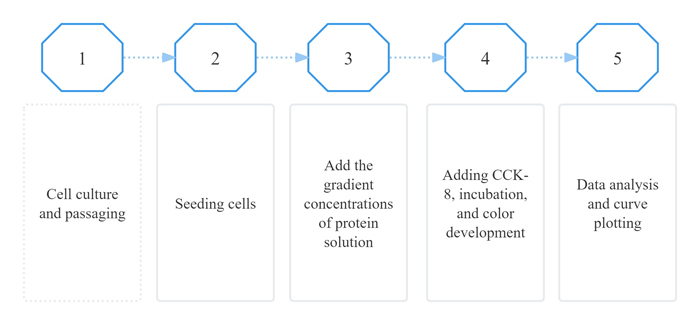
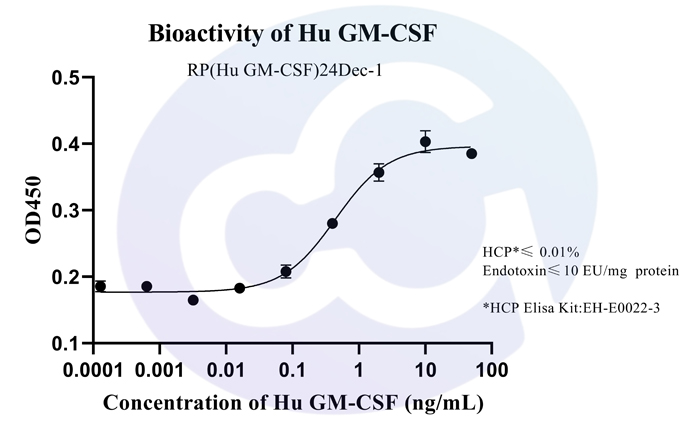
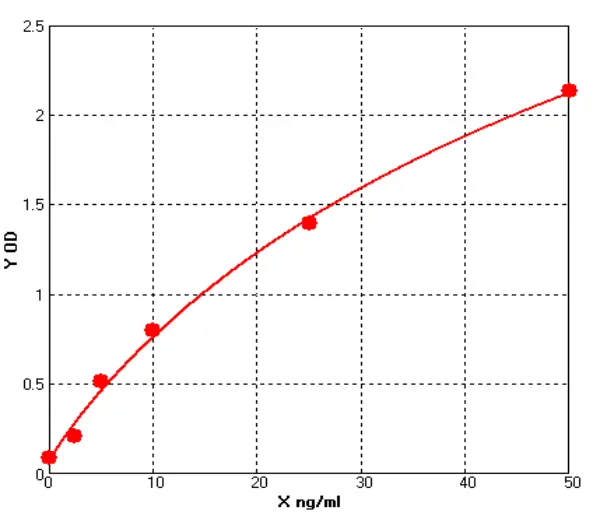
The biological activity of the protein was determined using TF-1 human erythroleukemia cells in a cell proliferation assay. The ED₅₀ was measured at 0.16–1.68 ng/mL, corresponding to a specific activity of 4 × 10⁶–4.2 × 10⁷ unit/mg, demonstrating excellent lot-to-lot consistency.
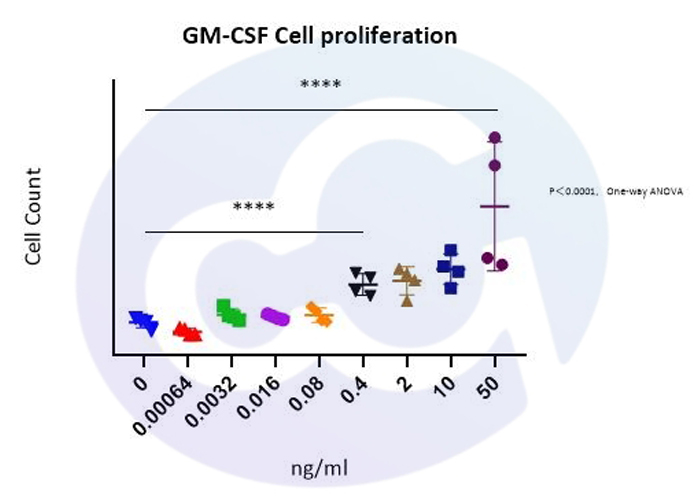
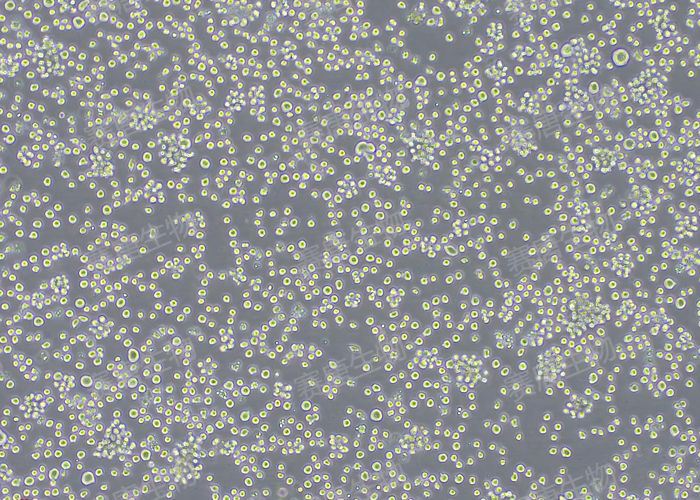
At varying protein concentration gradients, significant differences in TF-1 cell counts were observed. In addition, Hu GM-CSF can be supplemented as a growth factor in the basal culture medium of TF-1 cells, rapidly promoting cell proliferation. The cell morphology is shown below:
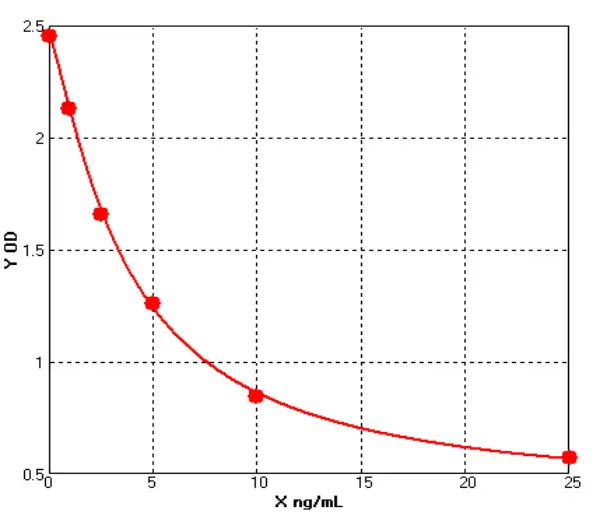
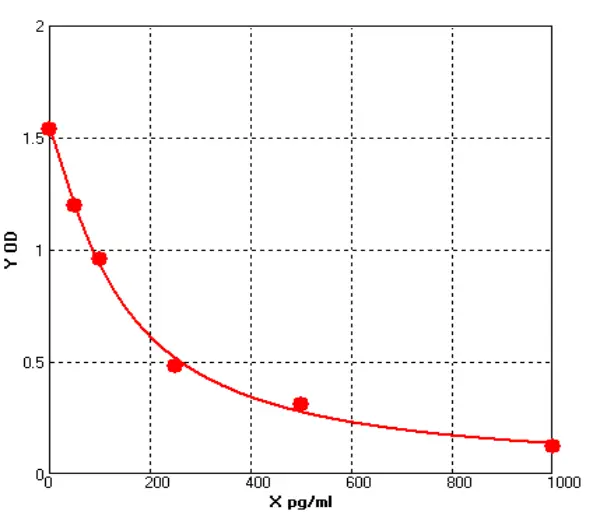
A comparative study was conducted with domestically available recombinant proteins, following a standardized SOP for all experimental procedures. The analysis results are as follows:
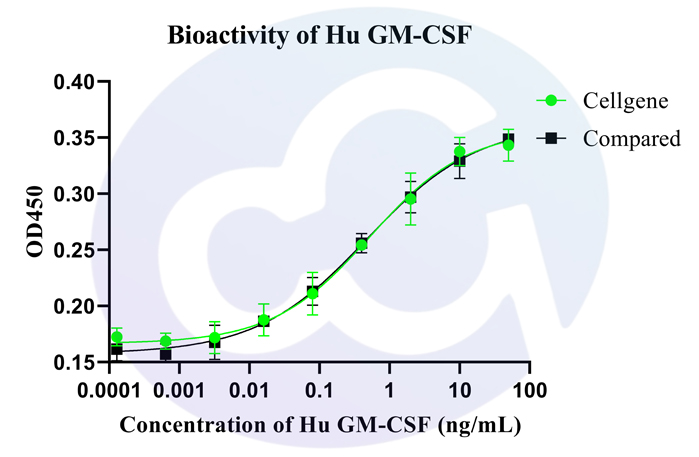
Based on four-parameter logistic (4PL) regression analysis, the ED₅₀ of the Seiton protein was 0.528 ng/mL, while that of the competitor protein was 0.477 ng/mL, indicating comparable activity levels between the two.