Search ELISA Kits
Drug Residue Test Kits Types
-
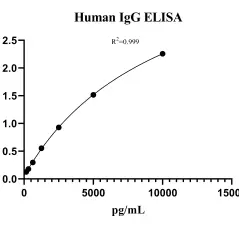
Human Immunoglobulin G (IgG) ELISA KitMANUAL
Cat. No.: NE01I0431
Detection Range: 156.250-10000 pg/ml
Reactivity: Human
Sensitivity: 10.2 pg/ml
-
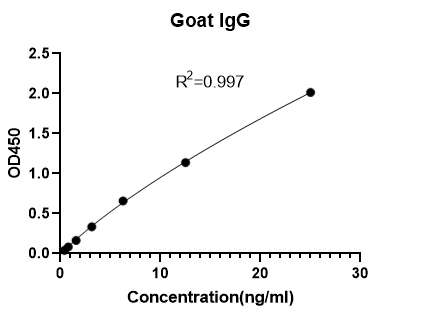
Goat Immunoglobulin G (IgG) ELISA KitMANUAL
Cat. No.:NE06I0431
Detection Range: 0.390625- 25ng/mL
Reactivity: Goat
Sensitivity: LOD: 0.05ng/mL; LOQ: 0.39ng/mL
-
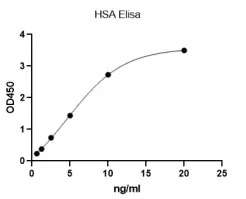
Human Serum Albumin (HSA) ELISA KitMANUAL
Cat. No.: NEGES0015
Detection Range: 0.625-20ng/mL
Reactivity: Human
Sensitivity: LOD:0.15ng/mL, LOQ: 0.625ng/mL
-
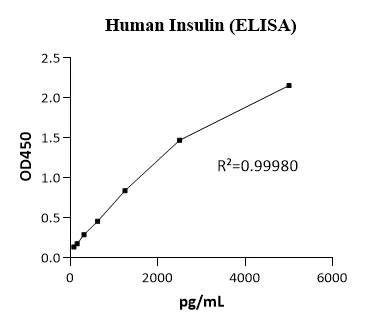
NE01I0004 Human Insulin (INS) ELISA Kit ( Double Antibody Sandwich Method )MANUAL
Cat. No.: NE01I0004
Detection Range: 78.125-5000pg/mL
Reactivity: Human
Sensitivity: LOD: 19.5pg/mL, LOQ: 78.125pg/mL
-
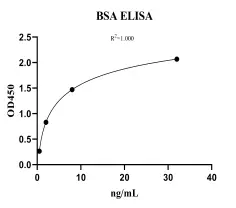
Bovine Serum Albumin (BSA) ELISA KitMANUAL
Cat. No.: NEGES0014
Detection Range: 0.5-32ng/ml
Reactivity: Bovine
Sensitivity: LOD:0.03ng/mL, LOQ: 0.5ng/mL
-
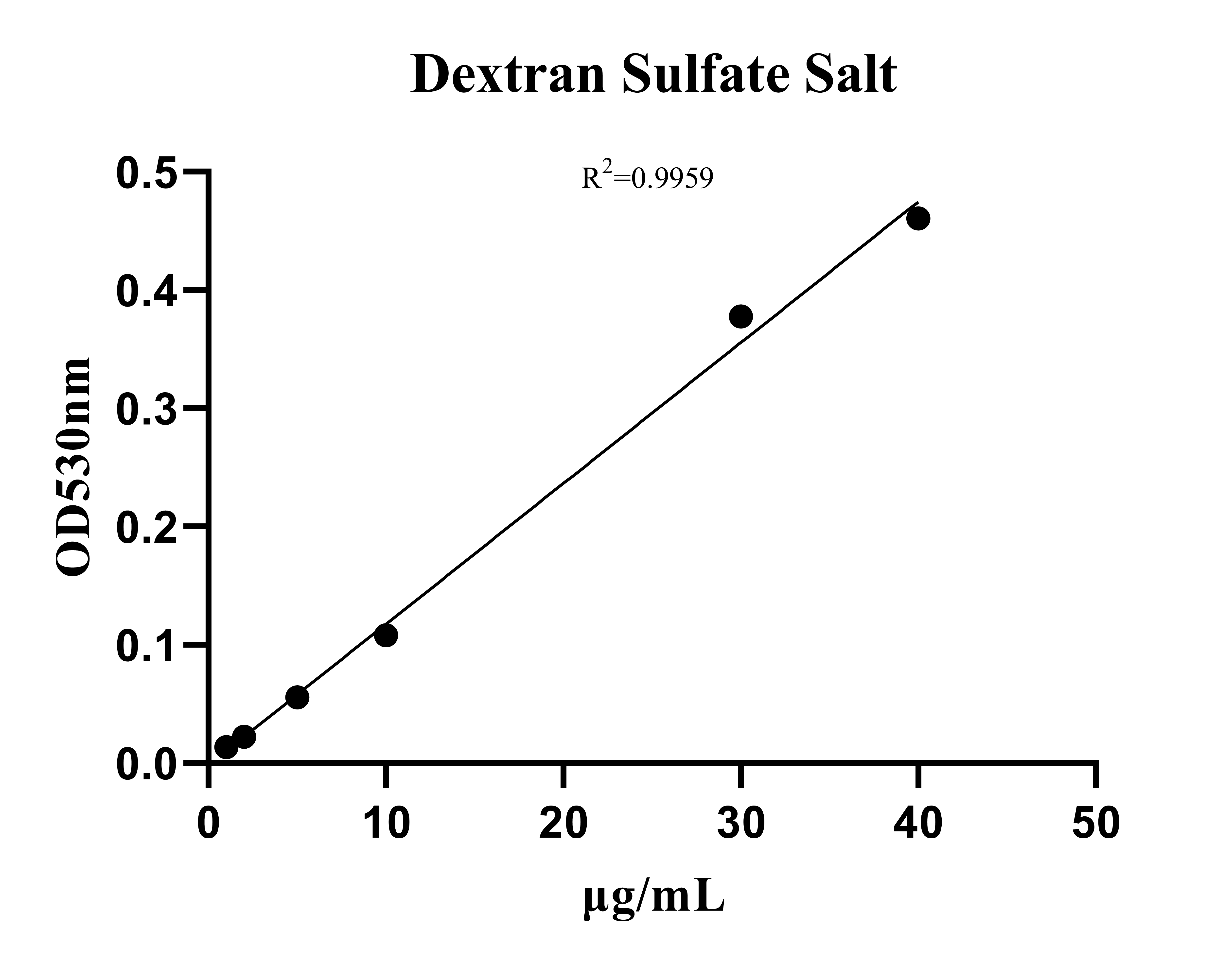
Dextran Sulfate Salt Detection Kit (Spectrophotometric Method-200 Tests)MANUAL
Cat. No.: NEGED0018
Detection Range: 1- 40ug/mL
Reactivity: Generic
Sensitivity: LOD: 1ug/mL, LOQ: 2ug/mL
-
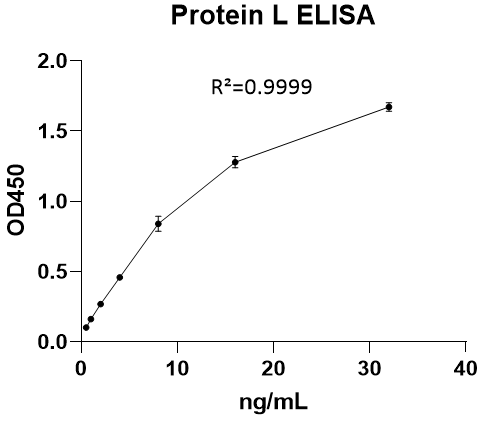
Protein L (PL) ELISA KitMANUAL
Cat. No.: NEGEP1270
Detection Range: 0.5- 32ng/mL
Reactivity: Generic
Sensitivity: , LOQ: 0.625ng/mL; LOQ: 0.5ng/mL
-
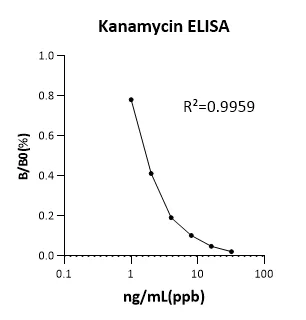
Kanamycin (KA) ELISA KitMANUAL
Cat. No.:NEGEK0006
Detection Range: 0- 32ng/mL
Reactivity: Generic
Sensitivity: LOD: 0.5ng/mL(ppb)
-
Human Immunoglobulin A (IgA) ELISA KitMANUAL
Cat. No.: NE01I0021
Detection Range: 0.097-6.25ng/mL
Reactivity: Human
Sensitivity: LOD: 3pg/mL, LOQ: 97pg/mL

-
Human Immunoglobulin M (IgM) ELISA KitMANUAL
Cat. No.: NE01I0038
Detection Range: 0.78-50ng/mL
Reactivity: Human
Sensitivity: LOD: 0.19ng/mL, LOQ: 0.78ng/mL

-
Mouse Immunoglobulin G (IgG) ELISA kitMANUAL
Cat. No.: NE03I0431
Detection Range: 78.125-5000 pg/ml
Reactivity: Mouse
Sensitivity: LOD: 9.7pg/mL; LOQ: 78.1pg/mL

-
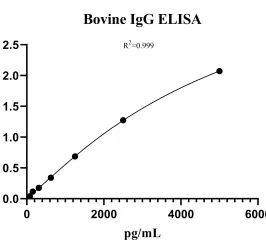
Bovine Immunoglobulin G (IgG) ELISA kitMANUAL
Cat. No.: NE11I0431
Detection Range: 78.125-5000 pg/ml
Reactivity: Bovine
Sensitivity: 26.8 pg/ml
-
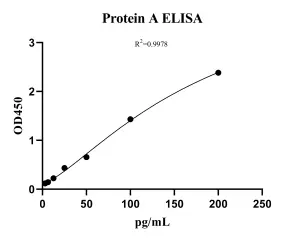
Protein A (PA) ELISA kit-BoilingMANUAL
Cat. No.: NEGES0890
Detection Range: 3.125-200 pg/ml
Reactivity: Generic
Sensitivity: 1.4pg/ml
-
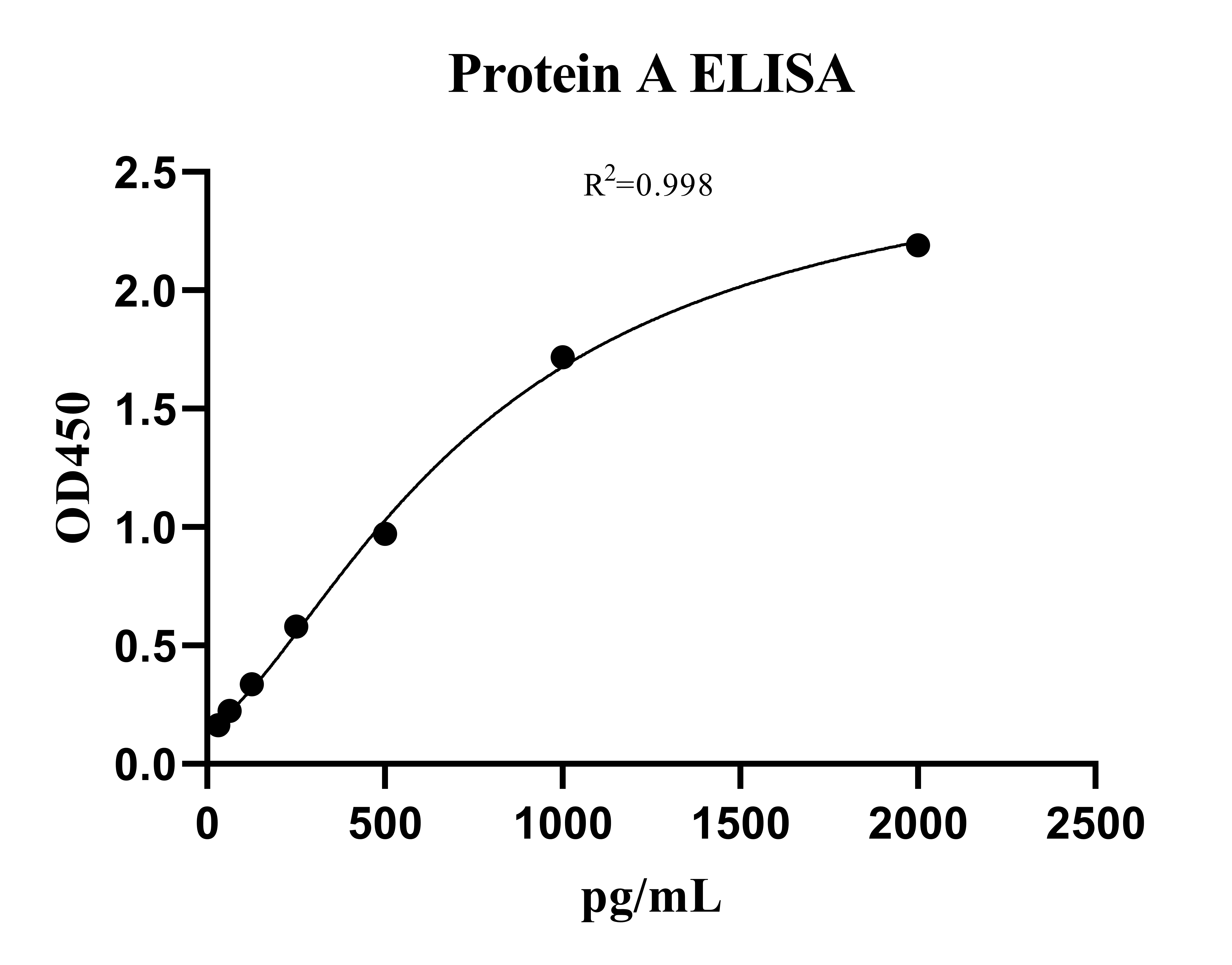
Protein A (PA) ELISA KitMANUAL
Cat. No.: NEGEP0890
Detection Range: 31.25- 2000pg/ml
Reactivity: Generic
Sensitivity: LOD: 3.9pg/mL; LOQ: 31.25pg/mL
Reliable Quality, End-to-End Control
• With 20 years of deep technical expertise, we possess a professional and efficient R&D team with extensive problem-solving experience.
• Equipped with professional 2D-AAE and LC-MS combined coverage validation analysis software, capable of providing coverage detection for antibody raw materials from different batches.
• Establish a complete electronic archiving system for research and development data. All data are electronically retained in accordance with standards, and the complete set of data required for customer audits can be provided at any time. Ensure that the data results are true and reliable, with full traceability throughout the process, and fully meet the requirements of audit compliance.
• Supported by sufficient strategic reserves of key raw materials to eliminate batch-change risks at source, our highly streamlined production system has demonstrated extreme stability across numerous historical batches, providing robust assurance for quality and efficiency.
• From R&D to production, we maintain complete autonomy over the entire process—including core technologies, the manufacturing system, and quality control. This integrated self-sufficiency guarantees reliable supply stability.
• Establish an ISO13485:2016 medical device quality management system, covering the entire lifecycle management of product development, production, delivery, and after-sales.
Introduction of lmmunoglobulin
Immunoglobulin is a group of globulins with a special chemical structure and immune function that exists in the body fluids and on the surface of lymphocytes, it is a material base for antibodies. The five major classes of immunoglobulin defined by their different structures and functions are IgG, IgM, IgA, IgD and IgE. Their molecular size, charge, amino acid composition and carbohydrate content are very uneven. Immunoglobulin molecule carries out dual tasks—binding to antigens and stimulating antibody production.