Yeast Family: Pichia pastoris (GS115) HCP ELISA
1. Application Background:
Pichia pastoris is one of the most widely used expression systems for innovative antibody expression in vaccines nowadays. Yeast is extensively applied in modern industry [1] and is inseparable from people's lives. It is commonly used in environmental remediation (bioremediation, pollutant degradation), biomanufacturing (crop protection/food and feed safety/probiotic production), biomedicine (drug discovery/drug resistance and metabolism/elucidation of disease mechanisms), fermentation (food fermentation such as beer and soy sauce), food and feed ingredients/raw materials (enzymes/edible flavors/edible pigments), biocatalysis (chiral chemical intermediates/biotransformation), as well as the cutting-edge modern application of heterologous protein production (drugs/hormones/vaccines)[2] (see Figure 1).
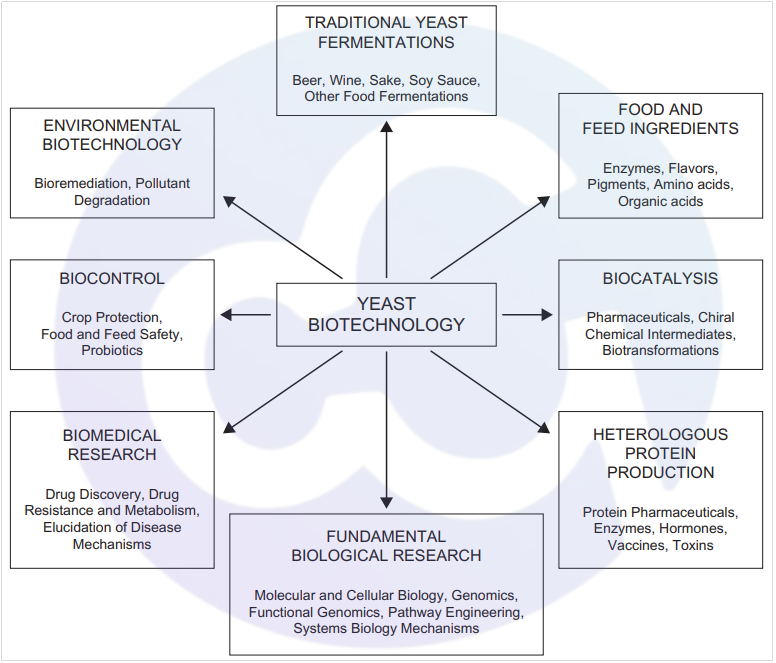
Figure 1
After over a century of modification and development, Pichia pastoris has gradually adapted to the expression of a wide range of pharmaceuticals. However, strains with different genotypes exhibit significant variations in the expression level, modification pattern, and resultant efficacy of these pharmaceuticals.
Pichia pastoris boasts a well-defined genetic background and demonstrates exceptional performance in protein folding and post-transcriptional glycosylation modification. In terms of oligosaccharide modification, each side chain of Pichia pastoris is modified with 8–14 mannose residues, which is substantially fewer than the 50–150 mannose residues found in Saccharomyces cerevisiae. Additionally, its O-linked glycosylation modification is minimal, potentially avoiding excessive glycosylation. Meanwhile, the presence of several key cofactors in Pichia pastoris has led to the evolution of its unique methanol metabolic pathway (see Figure 2). For these reasons, the Pichia pastoris expression system is widely adopted for the expression and production of certain pharmaceuticals [3,4,5,6]. Common pharmaceutical proteins expressed by Pichia pastoris are listed in Table 1 [7]:
Table 1
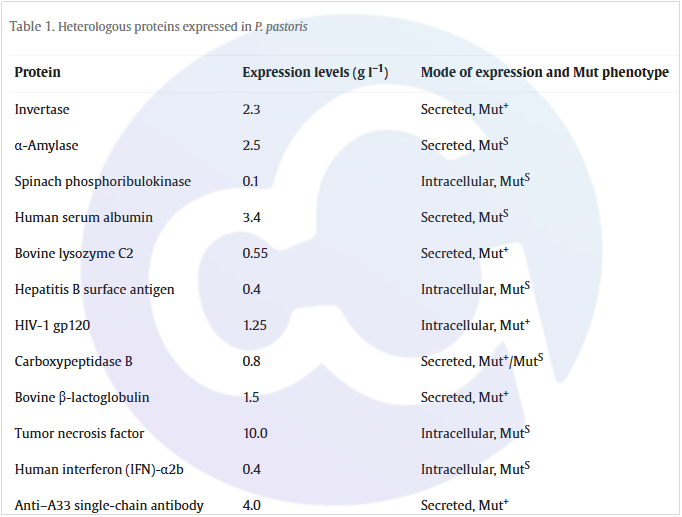
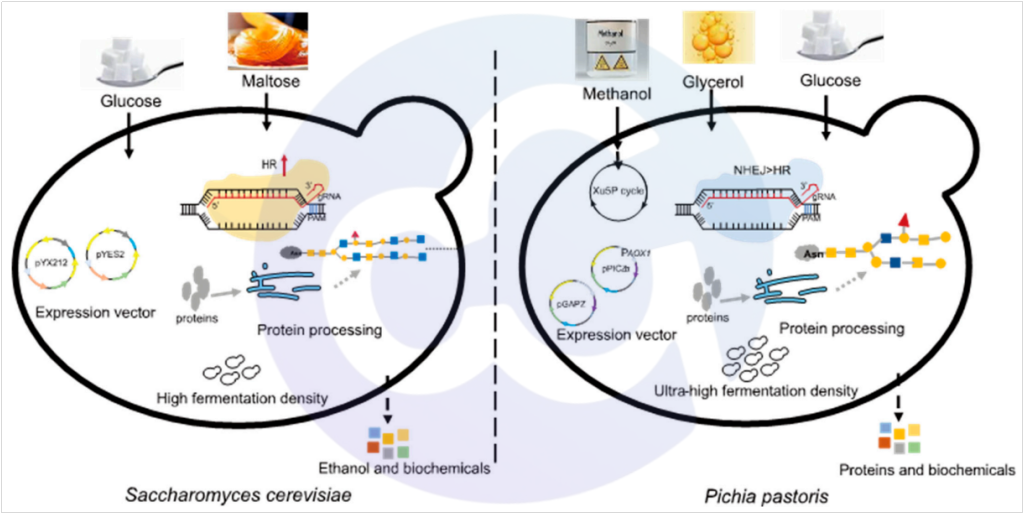
Figure 2
2. Genetic BackgroundAll Pichia pastoris strains are originally derived from the wild-type strain Y-11430 of the Northern Regional Research Laboratories (NRRL; Peoria, IL). The wild-type strain X-33 developed by Invitrogen was subjected to HIS4 gene mutation to generate the GS115 strain. Notably, GS115 is capable of growing in minimal medium without the addition of extra growth nutrients [8,9].。
Table 2
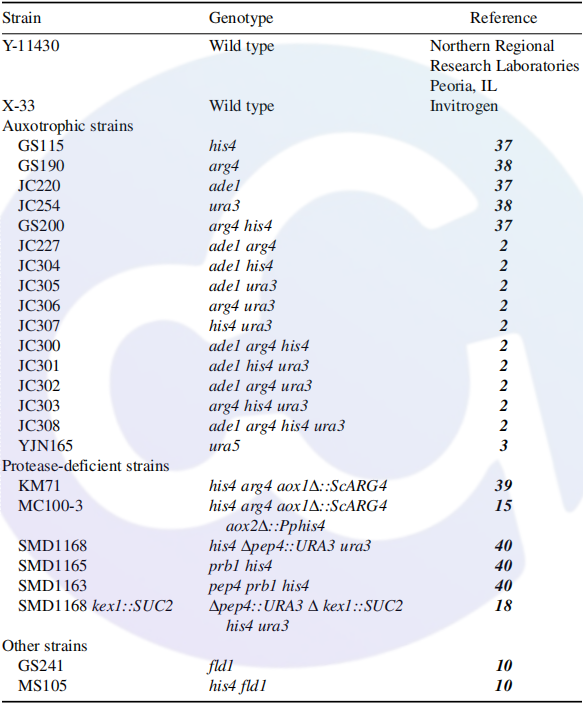
Currently, the most commonly used Pichia pastoris strains include K. phaffii X33 (Mut⁺, HIS4, AOX1, AOX2) and K. phaffii GS115 (Mut⁺, his4, AOX1, AOX2). In addition, X33 and GS115 have been engineered into multiple prevalent genetically modified variants, as listed below:
X33, AOX1: PAOX1-α-factor-hLALBA-TAOX1;
X33, AOX1: PAOX1-α-factor-hLALBA-TAOX1(6 copies);
GS115, ICL1: PICL1-LacI-Mit1AD-TAOX1;
GS115-ICL1-LM, his4: lacO-cPAOX1-α-factor-hLALBA-TAOX1;
GS115-ICL1-LM, his4: 5lacO-cPAOX1-P2-hLALBA-TAOX1, etc.[10]
Table 3
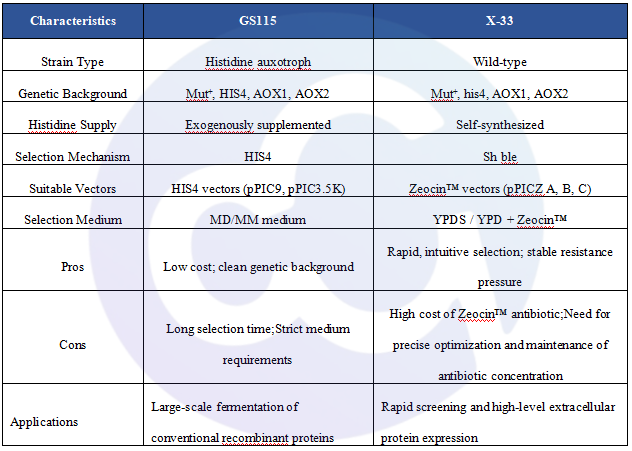
3. Quality Control Data: Whether it is Ogataea polymorpha, Saccharomyces cerevisiae, Pichia pastoris, or even the X-33 and GS115 strains derived from Pichia pastoris, despite their close genetic relationship, significant differences exist in their respective expression profiles. GS115 and X-33 vary distinctly in genetic background, selection markers, nutritional type, resistance, and application scenarios. Meanwhile, Host Cell Protein (HCP) residues in the diverse purification processes of different pharmaceuticals are even more incomparable.
In previous studies on yeasts, we have experimentally verified their cross-reactivity, blank background, Quantitation Limit (QL), anti-interference ability, and other performances. For details, refer to the following publications: "A Boon for Biopharmaceutical QC: High-Specificity Yeast HCP ELISA Panel Addresses Residual Detection Difficulties", "HCP ELISA Development: The Path to Background Optimization", and "Ogataea polymorpha HCP ELISA".
To meet the domestic and international needs for HCP detection of GS115 and its genetically modified derivative strains, we have developed a dedicated HCP ELISA Kit for GS115 Pichia pastoris (Catalog No.: PH-E0025-3A1). Comparative tests with the X-33 kit showed significant differences in detection results, indicating that GS115 and X-33 cannot be interchanged, either in terms of genetic background or sample detection performance.
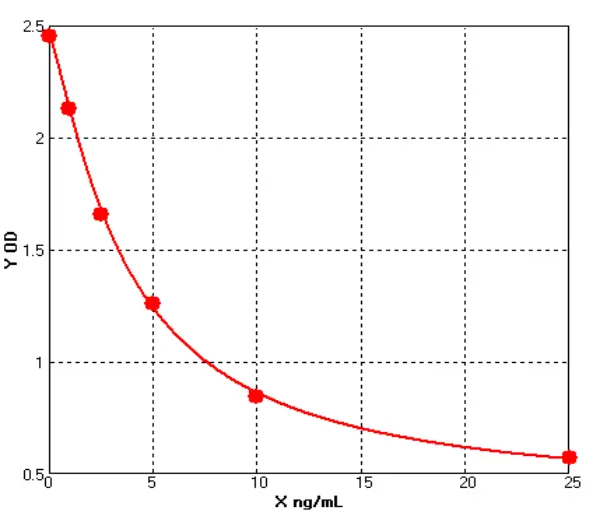
For Cellgene Bioscience's kit, the Detection Limit (DL) and Quantitation Limit (QL) are 0.39 ng/mL and 1.56 ng/mL, respectively (see Figure 3). The intra-batch and inter-batch Coefficient of Variation (CV) are controlled within 10%. The recovery rates under conditions containing Tris, Sodium Chloride, Urea, Isopropanol, Arginine, etc., are all within the range of 70%-130%, and most are within 80%-120%. These results demonstrate that the kit has significant advantages in sensitivity, precision, and recovery rate (see Tables 4 and 5).
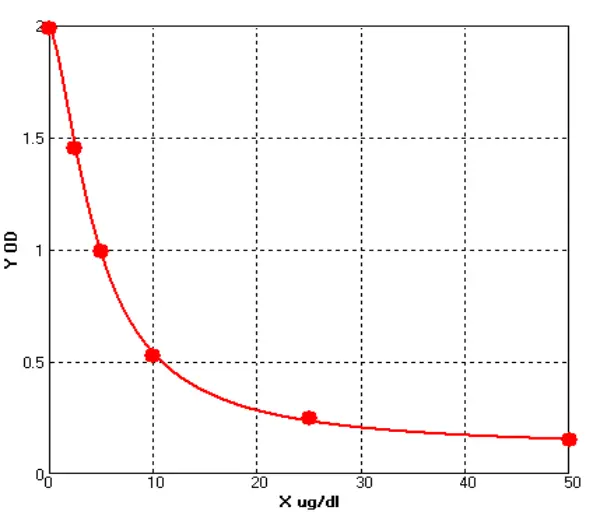
In addition, we compared PH-E0025-3A1 with Cygnus F1015. Both kits showed high HCP detection rates for the final drug products (see Figure 4).
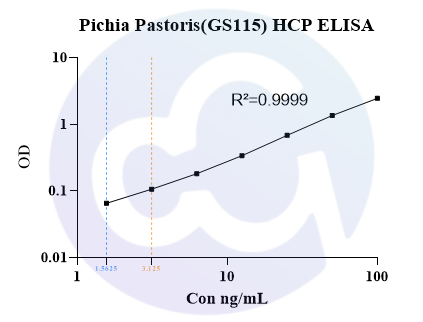
Figure 3
Note:The blue and orange lines represent the Detection Limit (DL) and Quantitation Limit (QL), respectively.
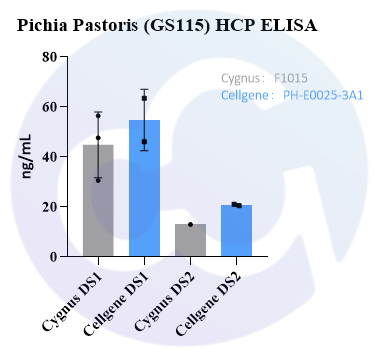
Figure 4
Table 4
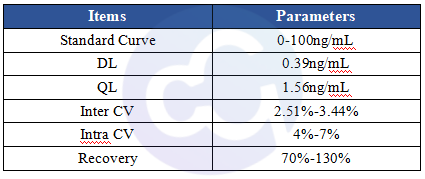
Table 5
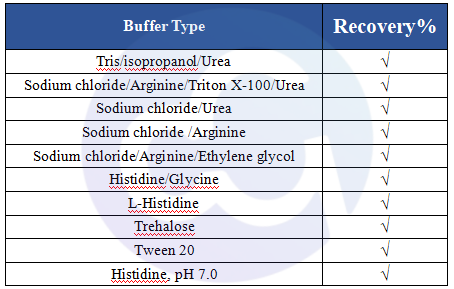
References:
[1]. Boud, D., & Walker, D. (1998). Promoting Reflection in Professional Courses: The Challenge of Context. Studies in Higher Education, 23, 191-206.
[2]. Cletus P. Kurtzman, et al. The Yeasts, a Taxonomic Study, Fifth Edition. Elsevier. 2011. 1:22.
[3]. Pan, Y.; Yang, J.; Wu, J.; Yang, L.; Fang, H. Current advances of Pichia pastoris as cell factories for production of recombinant proteins. Front. Microbiol. 2022, 13: 1059777.
[4]. Gao, J.; Jiang, L.; Lian, J. Development of synthetic biology tools to engineer Pichia pastoris as a chassis for the production of natural products. Synth. Syst. Biotechnol. 2021, 6, 110-119.
[5]. Grinna, L.S.; Tschopp, J.F. Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris. Yeast 1989, 5, 107-115.
[6]. Zhu, T.; Zhao, T.; Bankefa, O.E.; Li, Y. Engineering unnatural methylotrophic cell factories for methanol-based biomanufacturing: Challenges and opportunities. Biotechnol. Adv. 2020, 39: 107467.
[7]. C.A. Batt. Encyclopedia of Food Microbiology (Second Edition). 2014. 42-46.
[8]. Cregg, J. M., Barringer, K. J., Hessler, A. Y., and Madden, K. R. (1985) Pichia pastoris as a host system for transformations. Mol.Cell. Biol. 5, 3376-3385.
[9]. James M. Cregg, et al. Pichia Protocols, Second Edition. Methods in Molecular Biology. 2007. 389: 13.
[10]. Xinyi Wang, et al. Ethanol-Inducible Bioproduction of Human α-Lactalbumin in Komagataella phaffii. Agricultural and Food Chemistry. 2025. 73(15):9246-9260.
Cellgene Bioscience has been dedicated to the biopharmaceutical and industrial testing field for 15 years, offering a series of HCP residual detection products, as well as comprehensive technical services including HCP-specific antibody development and coverage analysis.
Cellgene Bioscience-Drug Residue Detection Products | |
Host Cell Protein ELISA kits (HCP) | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G2 | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G3 | |
HEK293 Host Cell Protein (HEK293 HCP) ELISA kit, G2 | |
E. coli Host Cell Protein (E. coli HCP) ELISA kit, G3 | |
Pichia pastoris Host Cell Protein (PP HCP) ELISA kit, G3 | |
Ogataea polymorpha Host Cell Protein ELISA kit, G3 | |
Saccharomyces cerevisiae Host Cell Protein ELISA kit, G3 | |
Spodoptera frugiperda (Sf9) Host Cell Protein ELISA kit, G3 | |
Medium Residues Detection kits | |
Protein A ELISA Kit (Boiling) | |
Protein A ELISA Kit | |
Mouse Immunoglobulin G ELISA Kit | |
Bovine Immunoglobulin G ELISA Kit | |
Human Immunoglobulin G ELISA Kit | |
Goat Immunoglobulin G ELISA Kit | |
Kanamycin ELISA Kit | |
Protein L ELISA Kit | |
NEGEP1271 | Protein G ELISA Kit |
Bovine Serum Albumin ELISA Kit | |
Human Serum Albumin ELISA Kit | |
Dextran Sulfate Salt Detection kit | |
Host Cell DNA Detection kits (HCD) | |
NS0 Host Cell DNA (NS0 HCD) Residue Detection kit | |
E.coli Host Cell DNA (E.coli HCD) Residue Detection kit | |
Vero Host Cell DNA (Vero HCD) Residue Detection kit | |
HEK293 Host Cell DNA (HEK293 HCD) Residue Detection kit | |
CHO Host Cell DNA (CHO HCD) Residue Detection Kit | |
Pichia Pastoris Host Cell DNA (PP HCD) Residue Detection Kit | |
Magnetic Residual DNA Sample Preparation Kit | |
Residual Total RNA Detection Kit | |
E.coli Residual Total RNA Detection Kit (qRT-PCR) | |
Host Cell Protein Antibodies | |
CHO Host Cell Protein G3 Antibody | |
CHO Host Cell Protein G2 Antibody | |
E.coli Host Cell Protein G3 Antibody | |
PH-E0021-2-Ab | Pichia Yeast Host Cell Protein G2 Antibody |
HEK293 Host Cell Protein G2 Antibody | |
Buffer Products | |
CG-H0100 | HCP ELISA buffer |
Protein L ELISA buffer | |