Building a Quality & Safety Ecosystem for Biopharmaceuticals
Background:
Quality and safety regulations for Cell and Gene Therapy (CGT) drugs are becoming increasingly globalized, with converging standards across China, the U.S., Europe, and Japan. Major economies worldwide are now enhancing monitoring and detection of impurities involved in regional drug production and clinical trials.
Against the backdrop of divergent development models among key economies and potential future risks, China’s biopharmaceutical industry must consolidate its supply chain and strengthen efficient upstream-downstream collaboration—while seeking common ground with global standards. For deeper insights, read Import Costs Surge: How Cellgene Bioscience Rises from "Alternative" to "Must-Have" and Reject Inefficient Involution: How Chinese Pharma Breaks Through in the Global Quality Discourse Battle.
Regulators including China’s NMPA and the U.S. FDA stipulate: Universal Host Cell Protein (HCP) detection kits are applicable for quantification in preclinical stages, but process-locked, characteristic HCPs require custom-specific HCP antibodies during clinical phases.
To standardize drug development and boost clinical success rates & safety of biopharmaceuticals, China’s CDE has further tightened impurity monitoring (with HCPs as a key focus) following the 2025 release of the new Chinese Pharmacopoeia—mandating HCP content testing at every purification step. Stricter approval processes for CGT and other drugs are now inevitable.
Outlook:
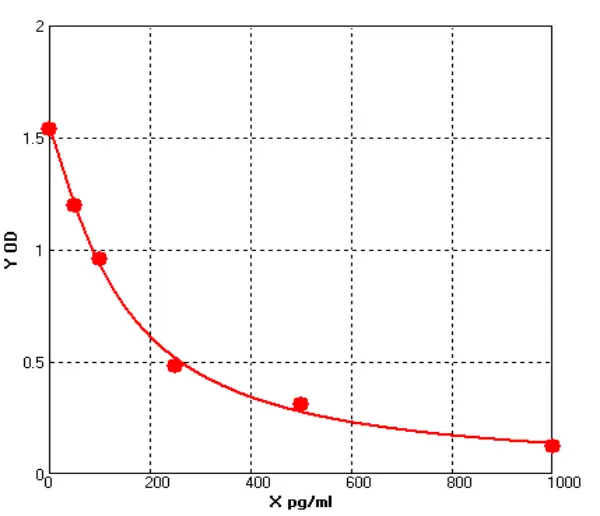
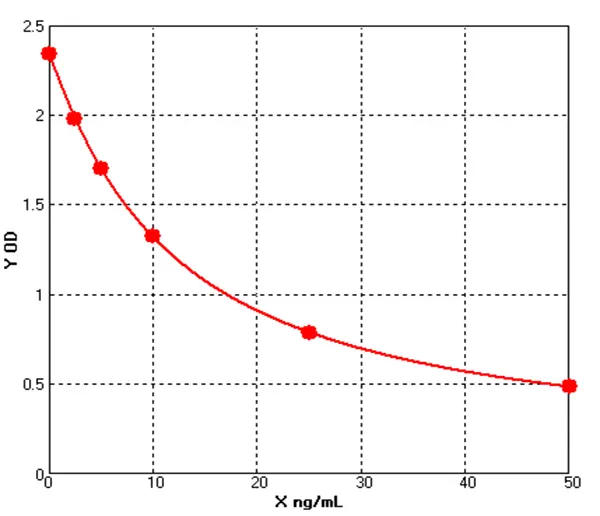
With 15 years of R&D experience in core impurity HCP detection, Cellgene Bioscience has launched a full-spectrum portfolio of detection products for biological macromolecules - covering total protein, total DNA, total RNA, small-molecule compounds, and other key impurities. The company has built a robust quality & safety detection ecosystem for biopharmaceuticals, centered on: HCP ELISA & HCD products for three major expression systems, mycoplasma detection via QPCR, and HCP core marker testing (Cathepsin D/B, PLBL2). This core range is complemented by peripheral HCP protein/peptide immunostimulatory marker detection systems (IFNγ/TNF ELISA, ELISpot, LFIA) (see Figure 1).
Today, Cellgene’s flagship HCP detection products cover all major expression systems for global biopharmaceuticals. Notably, its HCP detection solutions for Saccharomyces cerevisiae, Pichia pastoris, and Hi-5 cells—tailored for niche markets like vaccines—are exclusive to China.
Figure 1 Impurity Types
Types of Risk Factors
3.1 Host-Derived
3.1.1 Host cell protein (HCP):
Drug expression systems fall into four major categories:
1)Mammalian Expression Systems: Mouse-derived (CHO); Monkey-derived (Vero); Human-derived (293T, 293, MRC5)
2)Prokaryotic Expression Systems: E.coli (3S), E.coli (6S)
3)Fungal Expression Systems: Pichia pastoris, Pichia pastoris X-33, Pichia pastoris GS115, Saccharomyces cerevisiae
4)Insect Expression Systems: Sf9, Hi-5
Cellgene Biosciencehas systematically conducted HCP characterization and developed quantitative detection products for these systems. For detailed information, refer to Comprehensive Analysis of Host Cell Protein (HCP)G3 Detection Kit in Chinese Hamster Ovary (CHO) Cells, A Boon for Biopharmaceutical QC: High-Specificity Yeast HCP ELISA Panel Addresses Residual Detection Difficulties, HCP ELISA Development: The Path to Background Optimization, Ogataea polymorpha HCP ELISA, Performance Comparison of E.coli HCP ELISA Kits, Performance Comparison of HCP Products Between Cellgene and Cygnus.
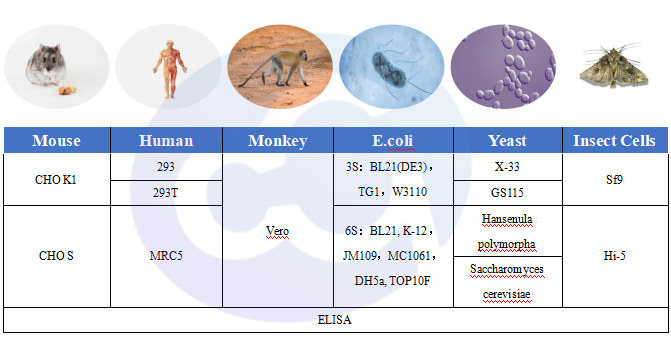
Figure 2 Expression System Host
3.1.2 Host cell DNA (HCD):
For drug sample processing, combined with magnetic bead-based pre-processing kits, it is suitable for DNA purification of 3 types of host expression systems:
1)Mammalian Expression Systems: Mouse-derived (CHO, NS0); Monkey-derived (Vero); Human-derived (293);
2)Prokaryotic Expression Systems: E.coli;
3)Fungal Expression Systems: Pichia pastoris, Saccharomyces cerevisiae.
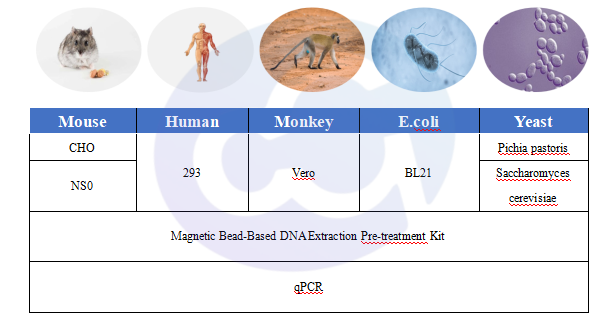
Figure 3 Host Cell DNA
3.1.3 Host cell RNA:
Exogenous nucleic acid contamination from host cells (including DNA and RNA) is subject to explicit testing requirements for cell-derived impurities imposed by the FDA, EMA, and NMPA. The total RNA product is E. coli RNA. For relevant technical documents, please refer to “E.coli Residual Total RNA Detection Kit (qRT-PCR)–Significance and Regulatory Context”
3.2 High Risk Protein:
In addition to monitoring total HCPs, common high-risk contaminant proteins derived from HCPs and in-process streams are also essential targets for mandatory monitoring. These high-risk contaminant proteins can be roughly classified into three categories:
1) Host cell protein origin:Cathepsin D, Cathepsin B, PLBL2;
2) Column origin: Protein A, Protein G, Protein L;
3) Culture medium origin: BSA, HSA, IgG, IgA, IgM, TRF, INS,Kana,Gen. For relevant technical documents, please refer to “New Product Launch: Protein L ELISA Kit”
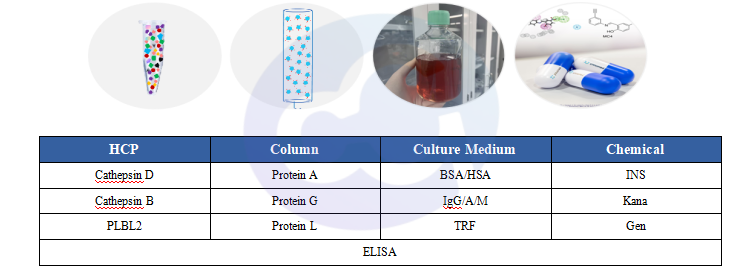
Figure 4 High-Risk Contaminant Categories
3.3 Immune Secretion
In the study of drug immunogenicity testing, immune stimulants in in vitro cell assays serve as critical evaluation indicators, especially cytokine secretion from human-sourced cells. To meet requirements for sensitivity and ease of use, Cellgene Bioscience offers two major categories of products:
1)Single indicator: SAA, CRP, hsCRP, IL10, TNFα, IFNγ, PCT;
2)Multi indicators: IFNγ/TNF, SAA/CRP, hsCRP/CRP, SAA/CRP/PCT/IL6, SAA/CRP/PCT/IL4;
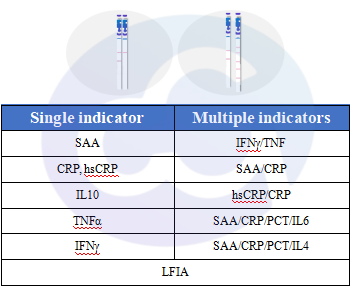
Figure 5 LFIA Series
3.4 Exogenous Microorganisms:
In cell and gene therapy (CGT) products, cell engineering and modification involve multiple steps, leading to a high risk of exogenous viral and microbial contamination. Compared with macromolecular antibody drugs, CGT drugs require more accurate, efficient, and sensitive detection methods and products to ensure drug safety. Cellgene Bioscience has developed two key kits:
a. Mycoplasma Detection Kit
b. Multiplex PCR Detection Kit for Mycoplasma/Chlamydia/SARS-CoV-2 (N/S/E Genes)/RSV.
Both are compatible with DNA extraction kits using magnetic bead-based or silica column-based methods. For detailed information, refer to “New Launch - Mycoplasma qPCR Detection Kit (Fluorescent Probe Method)”
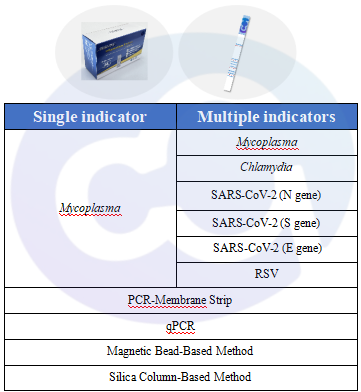
Figure 6 Exogenous Microorganisms
Detection Methods
Detection methods are diversified based on target types (single protein, multi-protein, nucleic acid, multi-nucleic acid, small-molecule hapten, etc.), as well as requirements for sensitivity and application scenarios. Details are as follows:
4.1 ELISA
ELISA (Enzyme-Linked Immunosorbent Assay) relies on the double-antibody sandwich antigen to form immune complexes. According to the difference in reaction signal intensity and sample types, it can be divided into polystyrene microplate ELISA and ELISpot.
The latter is mainly used for detecting trace cytokines released by cellular immune stimulation induced by drug immunogenicity. These cytokines are captured by pre-coated antibodies in the cell culture medium to form signal spots. Corresponding products include HCP and single-protein ELISA kits.
4.2 QPCR
For nucleic acid targets, Quantitative PCR (qPCR) is adopted, which combines specific primers and enzyme system components for quantitative analysis. Products cover host expression DNA, RNA, and exogenous microbial DNA0.
It is compatible with magnetic bead-based and silica membrane-based nucleic acid extraction methods. Corresponding products include HCD, HCR, and Mycoplasma detection kits.
4.3 LFIA
LFIA (Lateral Flow Immunoassay) is divided into antigen protein LFIA and PCR-LFIA. The former uses nitrocellulose membrane and glass fiber system for double-antibody sandwich antigen, and calculates results by reading the optical density data from gold particle accumulation in immune complexes. Corresponding products are test strip series.
PCR-LFIA combines PCR amplification system with LFIA, forming a visual and high-sensitivity method. Corresponding products include multi-pathogen test strips.
4.4 Electrochemiluminescence Assay
Residual proteins in hosts not only increase the risk of drug immunogenicity but also may catalyze the degradation of drugs and excipients. This method verifies the activity of highly relevant catalytic enzymes, which catalyze substrates to generate electrochemical signal changes for quantification. Corresponding products will be launched soon.
Supporting Platforms
Currently, relying on its technological accumulation and hardware/software development, Cellgene Bioscience is rooted in Zhangjiang (Gene Island) - a highland of China's pharmaceutical industry. It has established integrated cleanrooms for production and R&D (Figure 9), a standardized production quality management system (Figure 10), and upgraded the immunoassay platform (Figure 7) and PCR analysis platform (Figure 8) in a systematic manner.
Cellgene Bioscience boasts nearly 20 years of experience in ELISA technology and product development, as well as a 15-year R&D history in HCP products. Its HCP product portfolio has fully covered the expression systems of biological macromolecular drugs. The company will continue to focus on the field of biopharmaceutical quality, build an ecological system for biopharmaceutical quality and safety testing, and stably supply high-performance products for the global pharmaceutical industry.

Figure 7 Immunoassay Platform
Figure 8 PCR Analysis Platform

Figure 9 Cleanroom

Figure 10 Standardized System
Reference Standard:
ICH Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.
ICH Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.
ICH S6 (R1).
EMA: Guideline on Immunogenicity assessment of therapeutic proteins (2017).
FDA: Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products (1997).
NMPA: Chinese Pharmacopoeia (2025 Edition)
Cellgene Bioscience has been dedicated to the biopharmaceutical and industrial testing field for 15 years, offering a series of HCP residual detection products, as well as comprehensive technical services including HCP-specific antibody development and coverage analysis.
Product List
Cellgene Bioscience-Drug Residue Detection Products | |
Host Cell Protein ELISA kits (HCP) | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G2 | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G3 | |
HEK293 Host Cell Protein (HEK293 HCP) ELISA kit, G2 | |
E. coli Host Cell Protein (E. coli HCP) ELISA kit, G3 | |
Pichia pastoris Host Cell Protein (PP HCP) ELISA kit, G3 | |
Ogataea polymorpha Host Cell Protein ELISA kit, G3 | |
Saccharomyces cerevisiae Host Cell Protein ELISA kit, G3 | |
SF-H0025-3 | Spodoptera frugiperda (Sf9) Host Cell Protein ELISA kit, G3 |
Medium Residues Detection kits | |
Protein A ELISA Kit (Boiling) | |
Protein A ELISA Kit | |
Mouse Immunoglobulin G ELISA Kit | |
Bovine Immunoglobulin G ELISA Kit | |
Human Immunoglobulin G ELISA Kit | |
Goat Immunoglobulin G ELISA Kit | |
Kanamycin ELISA Kit | |
Protein L ELISA Kit | |
NEGEP1271 | Protein G ELISA Kit |
Bovine Serum Albumin ELISA Kit | |
Human Serum Albumin ELISA Kit | |
Dextran Sulfate Salt Detection kit | |
Host Cell DNA Detection kits (HCD) | |
NS0 Host Cell DNA (NS0 HCD) Residue Detection kit | |
E.coli Host Cell DNA (E.coli HCD) Residue Detection kit | |
Vero Host Cell DNA (Vero HCD) Residue Detection kit | |
HEK293 Host Cell DNA (HEK293 HCD) Residue Detection kit | |
CHO Host Cell DNA (CHO HCD) Residue Detection Kit | |
Pichia Pastoris Host Cell DNA (PP HCD) Residue Detection Kit | |
Magnetic Residual DNA Sample Preparation Kit | |
Residual Total RNA Detection Kit | |
E.coli Residual Total RNA Detection Kit (qRT-PCR) | |
Host Cell Protein Antibodies | |
CHO Host Cell Protein G3 Antibody | |
CHO Host Cell Protein G2 Antibody | |
E.coli Host Cell Protein G3 Antibody | |
PH-E0021-2-Ab | Pichia Yeast Host Cell Protein G2 Antibody |
HEK293 Host Cell Protein G2 Antibody | |
Buffer Products | |
CG-H0100 | HCP ELISA buffer |
Protein L ELISA buffer | |