A Boon for Biopharmaceutical QC: High-Specificity Yeast HCP ELISA Panel Addresses Residual Detection Difficulties.
Yeast is one of the most commonly used expression systems for biopharmaceutical macromolecules, including Saccharomyces cerevisiae, Pichia pastoris, and Kluyveromyces phaffii.
In accordance with the requirements of regulatory authorities such as the FDA, ICH, CDE, as well as the Chinese Pharmacopoeia and USP guidelines, and to meet the needs of pharmaceutical enterprises while addressing a gap in the market, Cellgene Bioscience has developed three dedicated Yeast HCP ELISA kits specific for each yeast species.
1. Background
1.1 Applications of the Yeast Expression System
The yeast expression system is one of the most widely used fungal expression systems. It is commonly employed in the production of traditional vaccines (e.g., hepatitis B, HPV), insulin and insulin analogs, antibody fragments (e.g., VHH), and glycoproteins (e.g., EPO). Compared to prokaryotic and mammalian cell expression systems, yeast offers distinct advantages, such as the ability to perform post-translational modifications (e.g., glycosylation), ease of genetic manipulation, low immunogenicity, and reduced production costs (see Table 1.1).
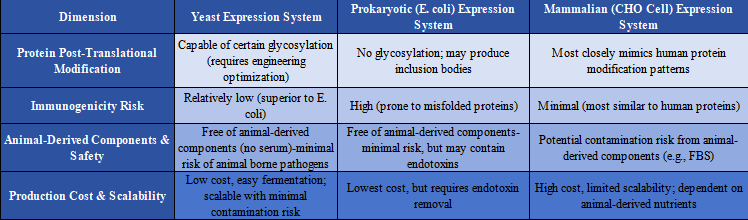
Table 1.1
Yeast provides efficient and effective glycosylation modification for proteins and can be classified into methylotrophic and non-methylotrophic species[2]. Non-methylotrophic yeasts include Saccharomyces cerevisiae strains: (S288c, A634A, BY4716, CEN.PK, ∑1278b, SK1, BJ5464, BY4742, and W303); Yarrowia lipolytica strains (W29, E150, E129, YB423, and CX161-1B). Methylotrophic yeasts include Pichia pastoris strains : (Wild-type Y-11430, X-33); Methanol utilization phenotypes: Mut⁺(AOX1+, AOX2⁺), Muts (AOX1⁻ , AOX2+), and Mut⁻ (AOX1⁻ , AOX2); Protease-deficient strains: SMD1163 (his4 pep4 prb1), SMD1165 (his4 prb1), and SMD1168 (his4 pep4); Histidinol dehydrogenase-deficient strains: GS115 (his4), KM71 (Δaox1::SARG4 his4 arg4), and SMD1168 (His4, pep4). Kluyveromyces phaffii strains: CBS4732 (CCY38-22-2, ATCC34438, NRRL-Y-5445), DL-1 (NRRL-Y-7560, ATCC26012), and NCYC495 (CBS1976, ATAA14754, NRRL-Y-1798) [1–3].
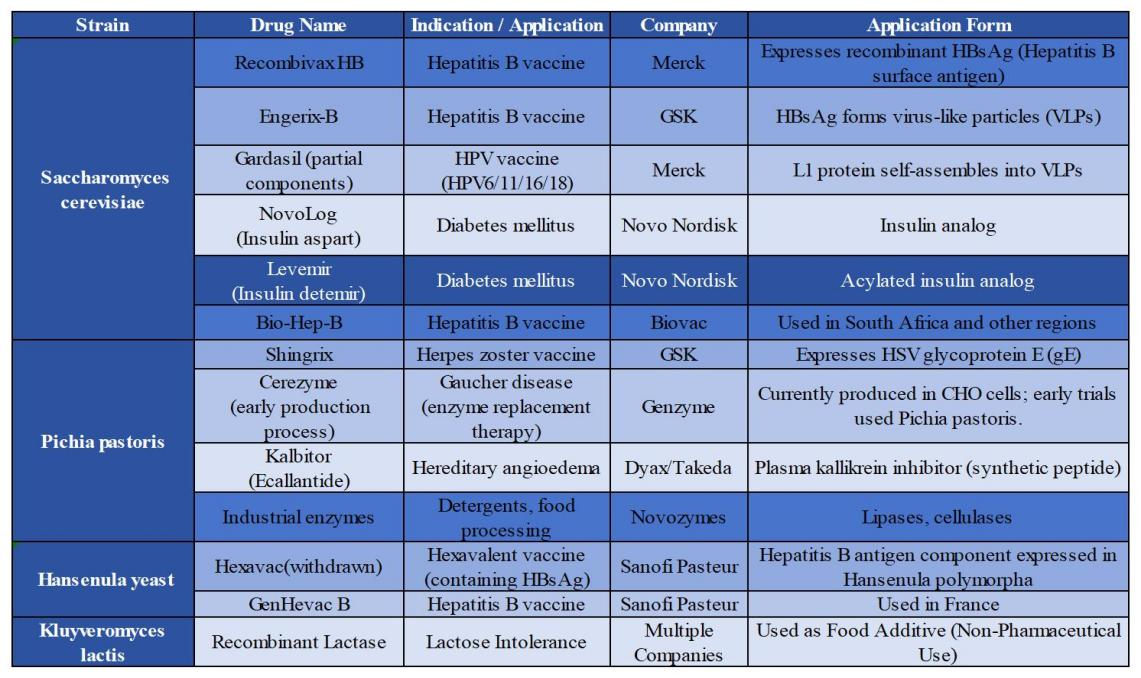
Table 1.2
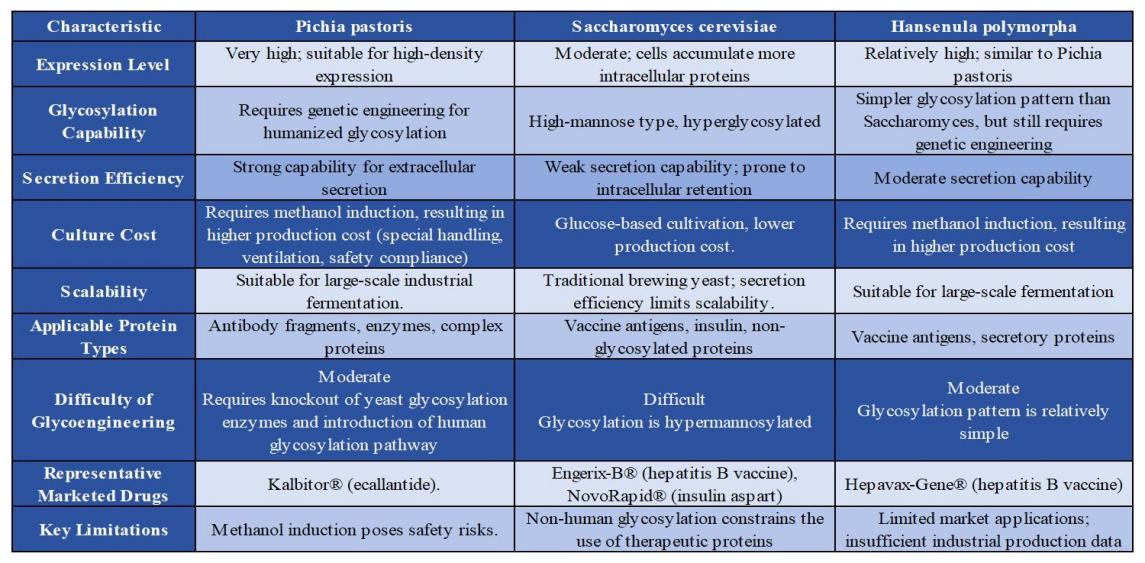
Table 1.3
1.1.1 Overview of the Pichia pastoris Expression System:
Methylotrophic yeast is a highly efficient protein expression system widely used in biopharmaceutical and industrial enzyme production. It offers advantages such as high cell density cultivation, ease of manipulation, rapid expression, low production cost, and the capability for post-translational modifications.
Taking Pichia pastoris as an example, this host features a methanol-inducible alcohol oxidase promoter (PAOX1), an efficient secretion pathway, robust post-transcriptional modification capabilities, and the ability to achieve high-density growth in specific culture media. The PAOX1 and AOX2 genes encode alcohol oxidase, a key enzyme in the methanol oxidation pathway. Through different mutation types, multiple strain variants can be derived. By utilizing the AOX1 promoter, methanol can serve as an inducer for high-level protein expression. Under optimized large-scale production conditions, enzyme yields can reach 20g/L.
Commonly used commercial Pichia pastoris strains include: GS115, X-33, Pichia Pink™, KM71. Protease-deficient strains: SMD1168 (his4, pep4, ::URA3, ura3), SMD1168H (pep4). Wild-type strains: BG10, X-33. Glycoengineered strains: SuperMan5 (HIS4+, Och1-disruption), SuperMan5 (HIS4+, pep4, Och1 disruption). Auxotrophic strains: PichiaPink™ (ade2), GS115 (his4). Common expression vectors include pJAN-s1 (BioGrammatics), pPICZ (Thermo Fisher Scientific), and pD902/pD905 (DNA2.0). Industrial enzymes produced in Pichia pastoris include Alkaline Xylanase (GS115), Neutral Protease I (GS115), α-Amylase (GS115), Lipase (GS115), Phytase (KM71), Laccase (GS115), β-Glucosidase (GS115), and Trypsin (GS115).
A major breakthrough in yeast-expressed therapeutic proteins came with FDA-approved drugs such as Jetrea® and Kalbitor®. Additionally, Pichia pastoris can be used to express Human Insulin (X-33), IgG (ScFv fragment) (X-33), HBsAg (GS115), Recombinant Human Interleukin-6 (X-33), Human Parathyroid Hormone (Methylotrophic Pichia strain), and Recombinant Human Erythropoietin (rhEPO) (X-33)etc. [5–7]
In contrast, non-methylotrophic Saccharomyces cerevisiae has produced several marketed recombinant protein therapeutics but suffers from limitations such as hyperglycosylation, low yield, and plasmid instability. As a result, CRISPR/Cas9 technology is introduced to induce mutations at specific sites. For example, in the GlycodExpress™ platform, sequential deletion of mannosyltransferase (MNN1) and other glycosyltransferase genes enhances N-glycan homogeneity and increases the transport capacity of UDP-GlcNAc in the Golgi apparatus[8].
1.1.2 Saccharomyces cerevisiae Expression System:
Taking Saccharomyces cerevisiae (baker’s yeast) as an example, its genome was the first eukaryotic genome fully sequenced in 1996. In industrial ethanol production, S. cerevisiae offers numerous advantages, including broad pH tolerance, high ethanol and sugar tolerance, and resistance to osmotic stress, outperforming bacteria, other yeasts, and filamentous fungi. It is also a widely used model for gene expression regulation and serves as a platform to study key biological processes such as signal transduction, aging, apoptosis, metabolism, cell cycle, programmed cell death, neurodegenerative diseases, autophagy, and secretion pathways.
However, heterologous glycoproteins produced in S. cerevisiae often undergo hypermannosylation, which may reduce protein activity and increase immunogenicity. Mannosylation is mainly mediated by Mnn2p and Mnn11p, while N-glycosylation can enhance protein secretion[10]. Knockout of α-1,6-mannosyltransferase Och1p enables production of active human tissue plasminogen activator.
1.1.3 Hansenula polymorpha Expression System
Hansenula polymorpha (methylotrophic yeast) can efficiently express heterologous proteins using strong inducible promoters such as FMD, MOX, and GAP. Its expression system supports glycosylation and allows high-density fermentation in fully defined synthetic media. With a well-characterized genetic background and high safety and efficiency, it has emerged as a promising eukaryotic expression system.
Key elements of H. polymorpha expression systems include:
· High-expression vectors for strong transcription of heterologous genes.
· Selectable markers, which are of two types:
Auxotrophic markers (e.g., URA3, LEU2, HIS4) that enable growth only in complementing media.
Dominant selection markers (e.g., antibiotic resistance genes G418, Zeocin).
Auxotrophic strains are typically generated through chemical mutagenesis, mating, or protoplast fusion.
Applications and Advantages of Yeast Expression Systems
Yeast expression platforms offer cost-effective and efficient hosts for biopharmaceutical production:
· Vaccines: e.g., hepatitis B vaccine (Engerix-B®) and HPV vaccine (Gardasil®), providing stable VLPs structures and enhanced immunogenicity.
· Insulin drugs: e.g., insulin aspart (NovoRapid®), offering reduced production costs at comparable efficacy.a
· Antibody fragments: e.g., Obinutuzumab (Gazyva®), Caplacizumab (Cablivi®), with glycoengineering improving ADCC and tissue penetration.
· Cytokines: e.g., Epoetin delta (Dynepo®), exhibiting extended half-life.
Overall, Pichia pastoris, S. cerevisiae, and H. polymorpha are widely applied in Asian pharmaceutical companies, but they differ significantly in expression level, secretion efficiency, glycosylation capacity, genetic manipulability, and suitability for different drug types. Comparative advantages and the yeast hosts used for marketed drugs are summarized in Tables 1.2 and 1.3.
2. Data
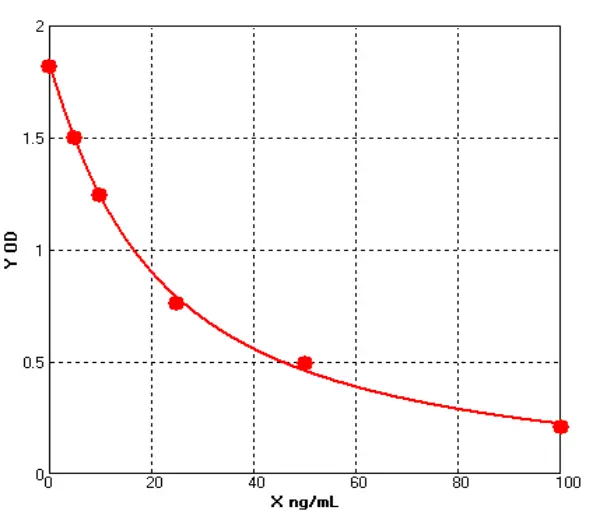
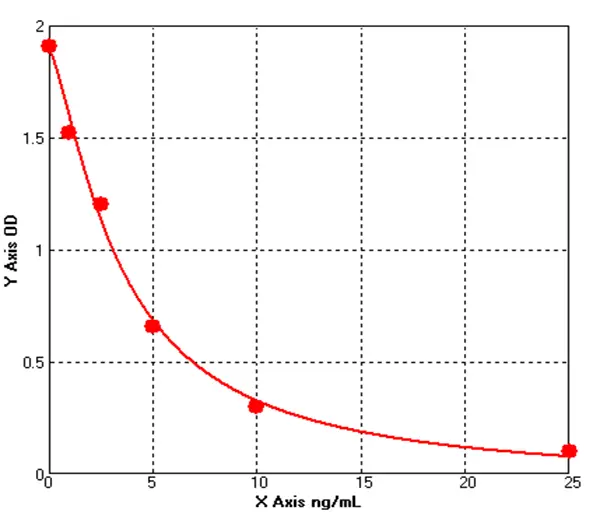
2.1 Standard Curve
For S. cerevisiae HCP, P. pastoris X-33 HCP, and H. polymorpha HCP ELISA assays, the 4-parameter logistic (4PL) regression fitting yielded R² values of 0.999 for all three, as shown in Figure 1.
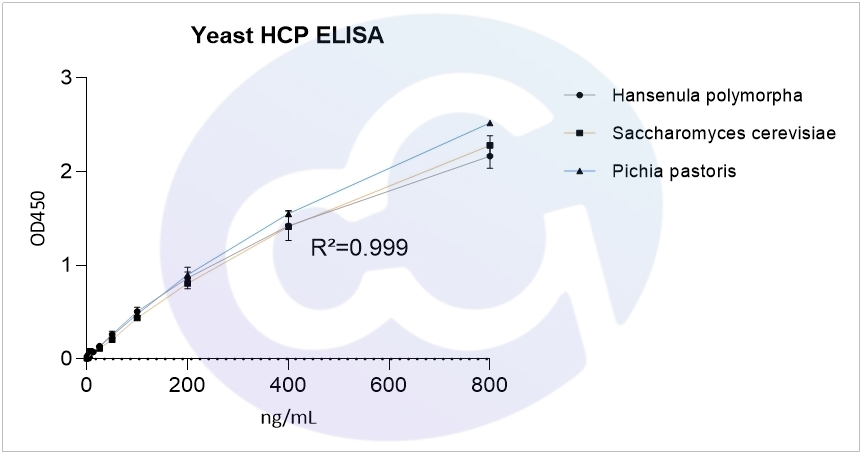
Figure 1
2.2 Quality Control Data
For P. pastoris X-33, H. polymorpha, and S. cerevisiae HCP assays, standard curve optimization was performed within the range of 0–800 ng/mL. The limit of detection (LOD) and limit of quantitation (LOQ) were determined for each assay. Cross-reactivity among the three yeast HCPs, as well as assay precision, were also evaluated. In addition, recovery rates under specific urea concentrations, salt concentrations, and pH conditions demonstrated excellent performance, as summarized in Table 2.1.
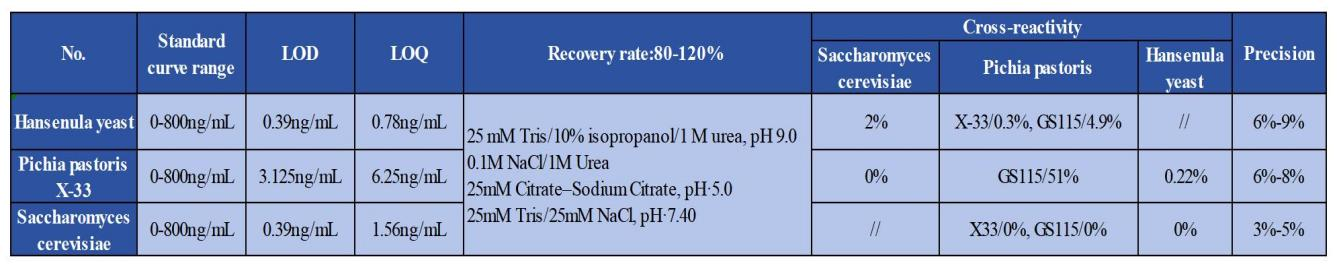
Table 2.1
Reference standard:
ICH Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.
ICH Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.
ICH S6 (R1).
EMA: Guideline on Immunogenicity assessment of therapeutic proteins (2017).
FDA: Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products (1997).
References:
[1] Fickers, P. Pichia pastoris: A workhorse for recombinant protein production[J]. Current Research in Microbiology and Biotechnology, 2014, 2(3):354-363.
[2] Vanz, A., Lünsdorf, H., Adnan, A., et al. Physiological response of Pichia pastoris GS115 to methanol-induced high level production of the Hepatitis B surface antigen: Catabolic adaptation, stress responses, and autophagic processes[J]. Microbial Cell Factories, 2012, 11(103):1-11.
[3] Stöckmann, C., Scheidle, M., Dittrich, B., et al. Process development in Hansenula polymorpha and Arxula adeninivorans, a reassessment[J]. Microbial Cell Factories, 2009, 8(22):1-10.
[4] Vieira, S. M., da Rocha, S. L. G., da Neves-Ferreira, A. G., et al. Heterologous expression of the anti myotoxic protein DM64 in Pichia pastoris[J]. PLoS Neglected Tropical Diseases, 2017,11(7):1-20.
[5] Meehl MA, Stadheim TA. Biopharmaceutical discovery and production in yeast[J]. Current opinion in biotechnology. 2014, 30:120-127.
[6] Thompson CA. FDA approves kallikrein inhibitor to treat hereditary angioedema[S]. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. 2010, 67(2):93.
[7] Imran Safder, Sajad Khan, Iram-us Islam, et al. Pichia pastoris expression system: a potential candidate to express protein in industrial and biopharmaceutical domains[J]. Biomedical Letters. 2018. 4(1):1-14.
[8] Piirainen, M. A., Boer, H., de Ruijter, J. C., et al. A dual approach for improving homogeneity of a human-type N-glycan structure in Saccharomyces cerevisiae[J]. Glycoconjugate Journal, 2016, 33(2):189-199.
[9] Tesfaw, A., & Assefa, F. Current trends in bioethanol production by Saccharomyces cerevisiae: Substrate, inhibitor reduction, growth variables, coculture, and immobilization[J]. International Scholarly Research Notices, 2014, 2014:1-11.
[10] Tang, H., Wang, S., Wang, J., et al. N-hypermannose glycosylation disruption enhances recombinant protein production by regulating secretory pathway and cell wall integrity in Saccharomyces cerevisiae[J]. Scientific Reports, 2016, 6(1):1-13.
Cellgene Bioscience has been dedicated to the biopharmaceutical and industrial testing field for 15 years, offering a series of HCP residual detection products, as well as comprehensive technical services including HCP-specific antibody development and coverage analysis.
Product Type:
Cellgene Bioscience-Drug Residue Products | |
Host Cell Protein ELISA kits (HCP) | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G2 | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G3 | |
HEK293 Host Cell Protein (HEK293 HCP) ELISA kit, G2 | |
E. coli Host Cell Protein (E. coli HCP) ELISA kit, G3 | |
Pichia Pastoris Host Cell Protein (PP HCP) ELISA kit, G3 | |
HP-H0023-3 | Hansenula Polymorpha Host Cell Protein ELISA kit, G3 |
SC-H0024-3 | Saccharomyces Cerevisiae Host Cell Protein ELISA kit, G3 |
SF-H0025-3 | Spodoptera Frugiperda 9 Host Cell Protein ELISA kit, G3 |
Medium Residues Detection kits | |
Protein A ELISA Kit (Boiling) | |
Protein A ELISA Kit | |
Mouse Immunoglobulin G ELISA Kit | |
Bovine Immunoglobulin G ELISA Kit | |
Human Immunoglobulin G ELISA Kit | |
Goat Immunoglobulin G ELISA Kit | |
Kanamycin ELISA Kit | |
Protein L ELISA Kit | |
NEGEP1271 | Protein G ELISA Kit |
Bovine Serum Albumin ELISA Kit | |
Human Serum Albumin ELISA Kit | |
Dextran Sulfate Salt Detection kit | |
Host Cell DNA Detection kits (HCD) | |
NS0 Host Cell DNA (NS0 HCD) Residue Detection kit | |
E.coli Host Cell DNA (E.coli HCD) Residue Detection kit | |
Vero Host Cell DNA (Vero HCD) Residue Detection kit | |
HEK293 Host Cell DNA (HEK293 HCD) Residue Detection kit | |
CHO Host Cell DNA (CHO HCD) Residue Detection Kit | |
Pichia Pastoris Host Cell DNA (PP HCD) Residue Detection Kit | |
Magnetic Residual DNA Sample Preparation Kit | |
Host Cell Protein Antibodies | |
CHO Host Cell Protein G3 Antibody | |
CHO Host Cell Protein G2 Antibody | |
E.coli Host Cell Protein G3 Antibody | |
PH-E0021-2-Ab | Pichia Yeast Host Cell Protein G2 Antibody |
HEK293 Host Cell Protein G2 Antibody | |
Buffer Products | |
CG-H0100 | HCP ELISA buffer |
Protein L ELISA buffer | |