Performance Comparison of HCP Products Between Cellgene and Cygnus
Background
Throughout the history of biopharmaceutical development, drug quality control and safety have always been paramount. With 40 years of experience in HCP products, Cygnus is undoubtedly the global leader in the HCP industry. Cellgene takes Cygnus as its benchmark. To systematically evaluate product performance, we conducted comparative tests using representative drug samples.
After 15 years of accumulation and development in HCP technology, Cellgene has achieved a qualitative leap in detection systems for multiple sample types. Collaborating with hundreds of biopharmaceutical companies, we selected typical drug samples from CHO and E. coli expression systems for comprehensive performance comparisons.
Results and Discussion
Given that E.coli and CHO HCP are the mainstream expression systems for global drug approval. We take CHO HCP as sample to compared Cellgene's products with this from Cygnus (USA). The product catalog numbers and HCP kit details are shown in the tables below.
2.1 Results
2.1.1 Drug Types and Catalog Numbers
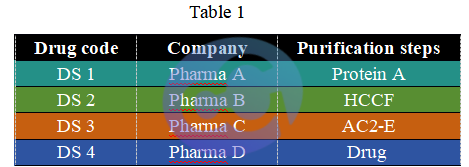
Note:
Protein A: Protein A purification of monoclonal antibody drugs; HCCF: Harvested cell culture fluid of antibody drugs; AC2-E: Eluted samples from Protein A and ion exchange chromatography of antibody drugs; Drug: Final purified product samples of antibody drugs.
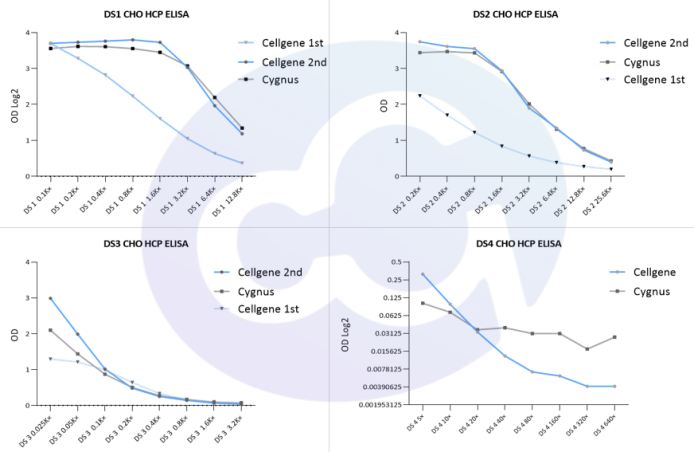
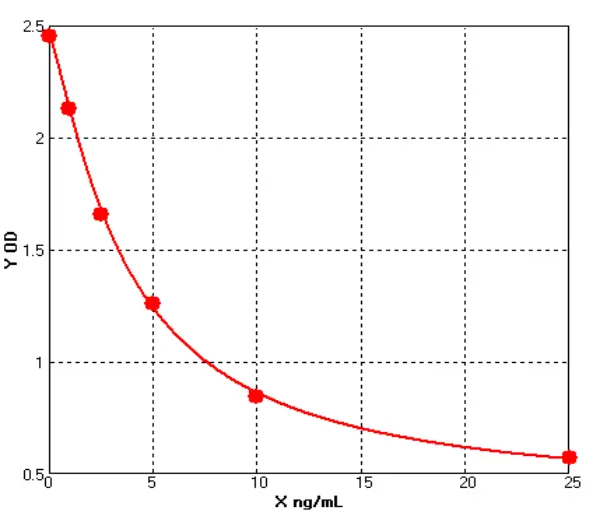
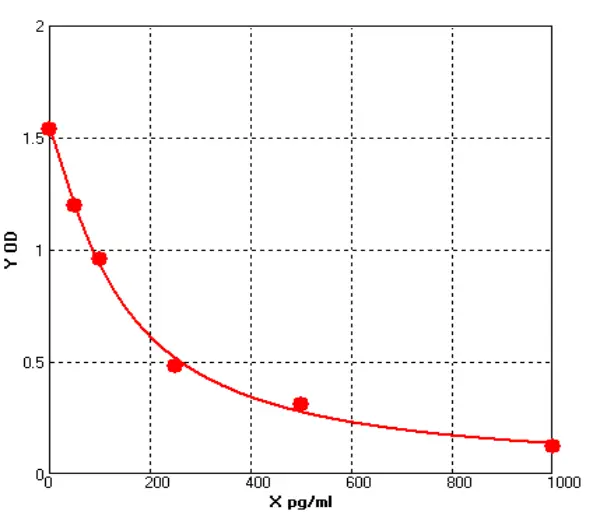
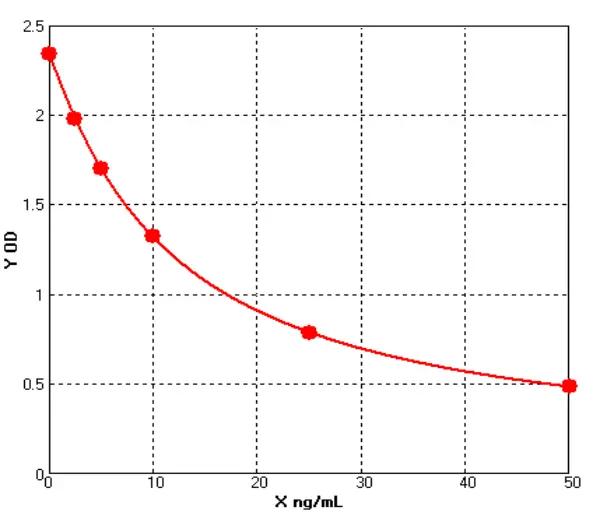
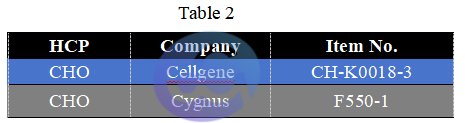
Figure 1
We selected representative samples from different drug purification stages and compared OD changes (representing concentration gradients) through serial dilutions under ELISA conditions. DS1 is the Protein A chromatography product of a customer's drug-gray represents Cygnus results, light blue represents our previous test results, and dark blue represents our latest results. DS2 is the HCCF product of another customer's drug. (see Figure 1).
Notably, our initial results showed a significant gap with Cygnus, but our latest data now approaches or even matches Cygnus' performance in some samples. For samples containing trace HCPs, Cellgene's ultra-low background, still exhibits a significant gradient decrease in OD after continuous dilutions in low-concentration samples, expanding the quantitative range.
Note: A systematic comparison of E.coli HCP products also yielded satisfactory results, as detailed in our previous article "Performance Comparison of E.coli HCP ELISA Kits".
2.1.2 Background Level
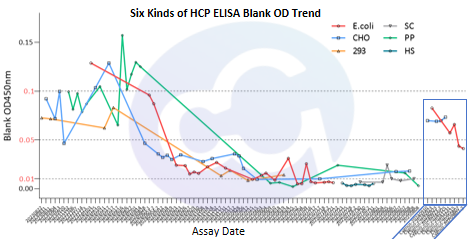
Figure 2
The blue box indicates Cygnus' background levels, mostly ranging from 0.05 to 0.09. Cellgene's HCP ELISA background has evolved continuously-from the initial 0.1, to 0.05, and now to 0.01, with internal quality control levels reaching 0.005-0.009. This fully reflects the continuous advancement of Cellgene's ELISA technology platform and our dedicated efforts in this niche field (see Figure 2).
Note: For details on background control, refer to "HCP ELISA Development: The Path to Background Optimization".
2.1.3 Assay Recovery Rate
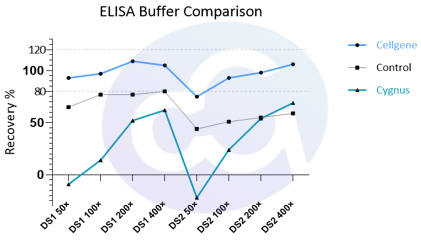
Figure 3
With technological advancements, biopharmaceuticals (recombinant proteins, monoclonal antibodies, bispecific antibodies, X antibodies, etc.), target types, drug buffer systems, and drug purification media are evolving rapidly, resulting in more dimensions of variation. This imposes increasingly stringent requirements on the interference stability of HCP ELISA detection.
We performed serial dilutions of drugs with extreme buffer systems selected from samples and compared ELISA recovery rates using Cellgene's proprietary HCP ELISA buffer. The results showed recovery rates could be controlled within 100±20% (actual quality control range: 100±30%). This enables the assay to adapt to more drug types and storage buffer systems, providing reliable data for HCP quantification during drug process development (see Figure 3).
2.1.4 High-Risk Proteins
Mass spectrometry comparison of HCP antibodies showed that Cellgene offers significantly better enrichment and coverage of common high-risk factor proteins compared to Cygnus, including lipoproteases, ribosomal proteins, S100 proteins, and transforming growth factors-key HCPs associated with drug safety and immunogenicity (see Figure 4).
Note: For technical details on mass spectrometry coverage, refer to Cellgene's HCPs white paper "Affinity Purification of Antibodies Using AAE for HCP Removal, Characterization, and Batch-to-Batch Quantification Stability".
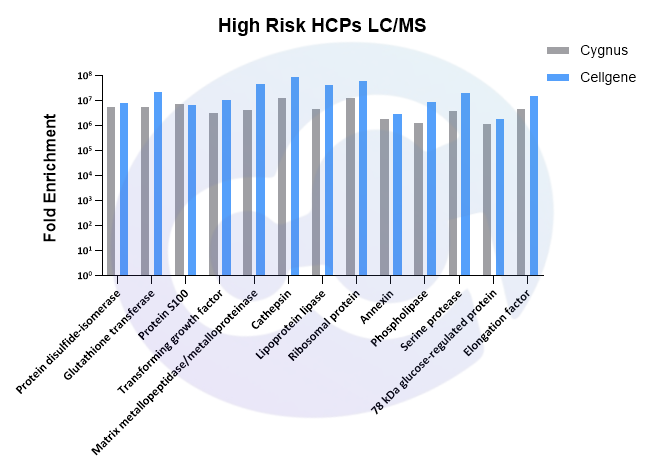
Figure 4
2.2 Discussion
We systematically compared our optimized HCP ELISA kits with Cygnus' products in terms of ELISA background, assay recovery rate, mass spectrometric coverage of HCP antibody enrichment, and serial dilution detection of typical purified products from clinical-stage drugs.
The comparison focused on three key aspects: linearity and concentration of independent sample dilutions, ELISA background, and recovery rate. Overall, our products offer lower background (reducing non-specific binding), a broader sample detection range, more accurate concentration measurements, and excellent recovery performance. For details on HCP ELISA development and quality control, refer to the datasheet of CHO Host Cell Protein ELISA Kit, G3(CH-K0018-3 ).
Cellgene's products meet Cygnus' performance standards across all parameters, with particular advantages in background control and recovery rate stability for samples with extreme buffers.
Cellgene adheres to a focused strategy: "Not a generalist, but a specialist; not pursuing breadth, but depth". We narrow our focus to quality control and anchor ourselves in biopharmaceutical upstream-downstream supply chain. With 20 years of expertise in ELISA, we will continue to deepen our commitment to the HCP quality control niche.
More HCP kits are coming soon!
Reference:
[1] ICH Q6B:Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.
[2] ICH Q7:Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.
Cellgene Bioscience has been dedicated to the biopharmaceutical and industrial testing field for 15 years, offering a series of HCP residual detection products, as well as comprehensive technical services including HCP-specific antibody development and coverage analysis.
Cellgene Bioscience-Drug Residue Detection Products | |
Host Cell Protein ELISA kits (HCP) | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G2 | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G3 | |
HEK293 Host Cell Protein (HEK293 HCP) ELISA kit, G2 | |
E. coli Host Cell Protein (E. coli HCP) ELISA kit, G3 | |
Pichia pastoris Host Cell Protein (PP HCP) ELISA kit, G3 | |
Ogataea polymorpha Host Cell Protein ELISA kit, G3 | |
Saccharomyces cerevisiae Host Cell Protein ELISA kit, G3 | |
SF-H0025-3 | Spodoptera frugiperda (Sf9) Host Cell Protein ELISA kit, G3 |
Medium Residues Detection kits | |
Protein A ELISA Kit (Boiling) | |
Protein A ELISA Kit | |
Mouse Immunoglobulin G ELISA Kit | |
Bovine Immunoglobulin G ELISA Kit | |
Human Immunoglobulin G ELISA Kit | |
Goat Immunoglobulin G ELISA Kit | |
Kanamycin ELISA Kit | |
Protein L ELISA Kit | |
NEGEP1271 | Protein G ELISA Kit |
Bovine Serum Albumin ELISA Kit | |
Human Serum Albumin ELISA Kit | |
Dextran Sulfate Salt Detection kit | |
Host Cell DNA Detection kits (HCD) | |
NS0 Host Cell DNA (NS0 HCD) Residue Detection kit | |
E.coli Host Cell DNA (E.coli HCD) Residue Detection kit | |
Vero Host Cell DNA (Vero HCD) Residue Detection kit | |
HEK293 Host Cell DNA (HEK293 HCD) Residue Detection kit | |
CHO Host Cell DNA (CHO HCD) Residue Detection Kit | |
Pichia Pastoris Host Cell DNA (PP HCD) Residue Detection Kit | |
Magnetic Residual DNA Sample Preparation Kit | |
Residual Total RNA Detection Kit | |
E.coli Residual Total RNA Detection Kit (qRT-PCR) | |
Host Cell Protein Antibodies | |
CHO Host Cell Protein G3 Antibody | |
CHO Host Cell Protein G2 Antibody | |
E.coli Host Cell Protein G3 Antibody | |
Pichia Yeast Host Cell Protein G2 Antibody | |
HEK293 Host Cell Protein G2 Antibody | |
Buffer Products | |
HCP ELISA buffer | |
Protein L ELISA buffer | |