HEK293 HCP ELISA: Fit for Viral Purification Processes, That’s What Truly Matters
Origin of HEK 293
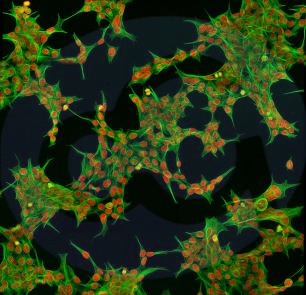
Human Embryonic Kidney 293 cells, commonly referred to as HEK 293, HEK-293, or 293 cells, are an immortalized cell line isolated from the embryo of a pregnant female in the 1970s[1].
In 1973, Alex van der Eb's laboratory in Leiden, the Netherlands, transfected adenovirus DNA into in vitro cultured 293 cells for the first time. In 1977, Frank Graham published a paper on HEK transfection at McMaster University. Since the HEK cells had been passaged 293 times at that time, they were named HEK293 cells.
A comprehensive study on the genome and transcriptome of the HEK 293 cell line and its five derivative cell lines compared the transcriptome of HEK 293 with those of human kidney, adrenal gland, pituitary gland, and central nervous system tissues. It was found that the properties of this cell are closer to those of the adrenal gland[2].
Figure 1 |
Figure 2 |
Expression Advantages
Sequencing verification has confirmed that the left-arm genome of adenovirus (approximately 4.5 kbp) is integrated into chromosome 19 of the cell[3].
Classification and Evolution
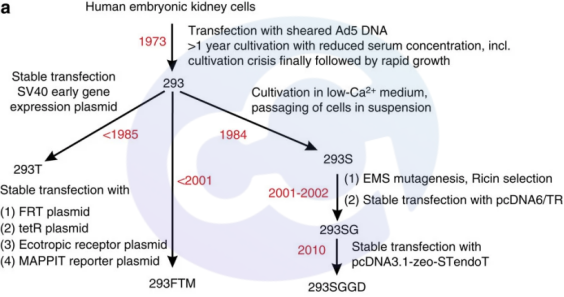
After years of development, HEK293 cells have evolved into a wide range of fibroblastic, endothelial, and epithelial cells derived from the kidney. The cell morphology is mostly triangular or spindle-shaped. Over decades of development, industrial and scientific communities have developed subtypes such as 293F, 293T, and 293S (see figure). As mentioned above:
· In 1973, HEK293 could be infected with Ad5 adenovirus for screening immortalized cells;
· In 1984, HEK293S achieved suspension growth in MEN medium;
· In 1985, a temperature-sensitive SV40 large T antigen cell line was screened, increasing the expression level of the SV40 promoter;
· In 1993, HEK293H grows rapidly in adherent state with high protein expression and is adapted to serum-free medium;
Subsequent derivatives include HEK293FT (a neo-resistant mutant cell capable of infecting lentivirus), HEK293F (optimized in 2014 for commercial large-scale culture), HEK293E (EBVA) and HEK2936E (the former expresses EBNA-1 protein, while the latter expresses EBNA1t protein with deleted Gly-Gly-Ala repeat region), HEK293FTM (2001, containing stably transfected FRT sites and TetR plasmids), HEK293SG (2002), HEK293SGGD (2010), HEK293A, and HEK293MSR. These modified cell lines are adapted to different functions and drugs, with modified target genes related to cell proliferation, apoptosis, central carbon metabolism, glycan profile homogeneity, glycosylation efficiency, protein folding, secretory system, and protein expression.
Advantage Comparison
Currently, the CHO expression system is still the most mature and widely used mammalian expression system. However, the 293 system has core advantages in rapid transient transfection, similarity of post-translational modifications to human species, and expression of large molecular protein complex particles. Therefore, the 293 system remains one of the most promising mammalian expression systems. A comparison of expression advantages with CHO is shown in Table 1 below:
Table 1
Comparison Dimension | HEK 293 Cells | CHO Cells |
Core Positioning | Rapid R&D and complex protein production | Gold standard for large-scale production |
Cell Origin | Human-derived | Murine-derived |
Core Advantages | Human-like post-translational modifications (glycosylation, γ-carboxylation, etc.);Production of viral vectors, membrane proteins, and multi-subunit complexes | Extremely high expression yield, mature process,Stable scalability, and directional optimization of glycosylation |
Disadvantages | 1. Lower yield of stable cell lines than CHO, higher cost for large-scale production; 2. Less mature suspension acclimation/clone screening platform than CHO; 3. Carries adenoviral gene fragments (E1), requiring safety assessment | 1. Incomplete humanization of post-translational modifications, which may produce immunogenic glycoforms (e.g., NGNA);2. Low transient expression efficiency and slow early R&D progress;3. Difficult or low-activity expression of certain complex human-derived proteins (especially membrane proteins) |
Application Scope | Membrane proteins/receptors (GPCRs);AAV/lentivirus/coagulation factors | Commercial antibody drugs/Fc fusion proteins/hormones/cytokines |
Process | Mainly transient transfection → Obtaining protein samples in 1-2 weeks → Developing stable cell lines if production is needed. | Mainly stable cell line development → Taking months to screen high-expression clones → Conducting process development and scale-up. |
Genetic Modification:
Due to the core advantages of 293, the scientific and industrial communities have continuously modified the 293 genome, focusing on functions such as cell apoptosis, cell proliferation, cellular central carbon metabolism, glycosylation, protein folding, and protein secretion. The main technical means include knockout and knockdown inhibition based on miRNA and siRNA, proteomic analysis, and high-throughput screening of suitable expression conditions (see Figure 3)
Figure 3
Genes with related functions include:
Cell cycle genes that enhance protein expression and promote G1-S transition, such as Cyclin-dependent kinase like 3 (CDKL3);
Genes that promote synthetic functions, such as Cytochrome c oxidase subunit 15 (COX15);
Cell growth-related genes that regulate G1 progression, such as Cyclin-dependent Kinase inhibitor 2C (CDKN2C);
Genes that inhibit the synthesis of anti-apoptotic proteins, such as B-cell lymphoma 2 (BCL2);
Dehydrogenase kinase (PDK), which reduces dehydrogenase kinase activity and increases the conversion of pyruvate to lactate in the cytoplasm;
MGAT1 gene, which knocks out high-mannose modification (high-frequency mannose modification increases the risk of drug modification and is more likely to be presented to immune cells, increasing the possibility of in vivo drug clearance).
Relevant gene types are shown in Table 2 below:
Table 2
Functions | Gene |
Proliferation | CDKL3, COX15, EIF3I, CDKN1A/B, CDKN2C, FGF1 |
Apoptosis | XIAP, BCL2, CASP3/6/7, AIF1, BAX/BAK, CrmA, NRF2 |
Central carbon metabolism | Carboxylase, Dehydrogenase, PDK, HIF1A |
Glycan pattern homogeneity Glycosylation efficiency | EndoT8/β-galactoside-α-2,6-sialyltransferase 1, MAN1A1/2, MAN1B1, FUT8,MAN1C1/MGAT1, Sialyl transferases, ST6GAL1, ST3GAL3/4, MAN2A1/2, |
Protein folding | RIC3, XBP1, PDIA2, Hsp70/HSPA5, VKORC1, CALU |
Secretory machinery | SNAP-23, VAMP8, MUNC18, sly1, Tetraspanin CD9 |
Protein expression | HIPK1, miRNA-22-3b, CEBPG, OAZ1, CASP8AP2, FABP5, RETNLB, PC, XBP1, HSPA5, HERPUD1, NEDD8/4L, UGCG, CIT, CNP |
HEK293 HCP ELISA Kit Developed by Cellgene Bioscience:
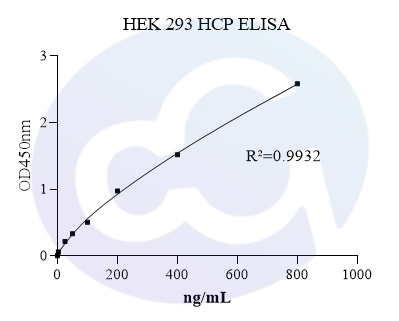
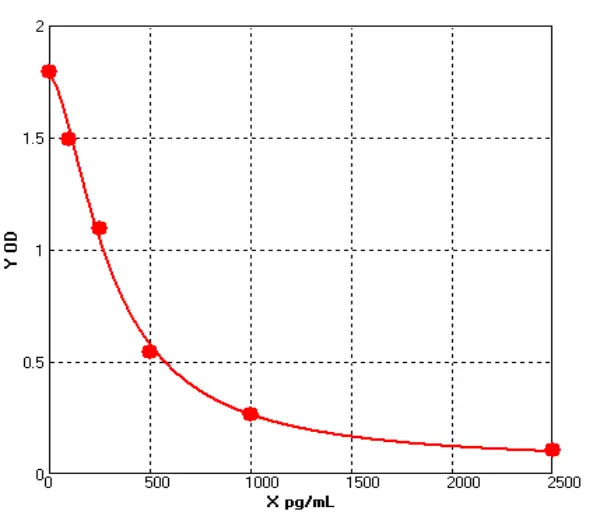
Based on the above functions and application potential of HEK293, Cellgene Bioscience has developed the HEK293 HCP ELISA (HH-H0019-3A1) Kit. The Detection Limit (DL) and Quantitation Limit (QL) of this kit can be controlled at 0.39 ng/mL and 0.78 ng/mL, with excellent linearity (80-120%). It has been confirmed applicable to samples such as recombinant monoclonal antibodies, bispecific antibodies, and viral packaging samples. The data are shown in Table 3 below:
![]()
Table 3
QC | Data source(2025-2026) | |||||
DL | 0.39ng/mL | |||||
QL | 0.78-800ng/mL | |||||
Linear | 70%-130% | Diluent | Mean OD | ng/mL | Con ng/mL | Recovery |
40× | 0.33 | 91.8 | 3670.4 | 96.32% | ||
80× | 0.168 | 41.0 | 3279.0 | 89.34% | ||
160× | 0.098 | 21.0 | 3364.2 | 102.60% | ||
320× | 0.063 | 11.8 | 3767.4 | 111.99% | ||
640× | 0.036 | 5.2 | 3312.7 | 87.93% | ||
Recovery | 70%-130%(8×Buffer) | |||||
CV(S2/S4/S6) | <3.0%-4.6% | |||||
Samples | mAb/BiAb/AAV | |||||
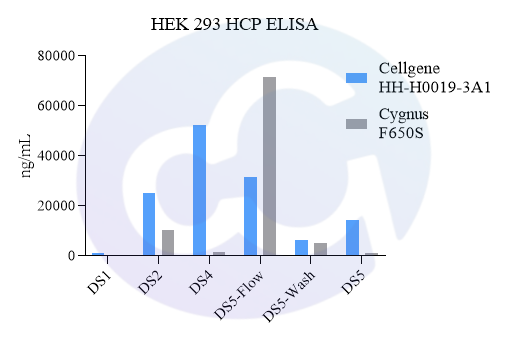
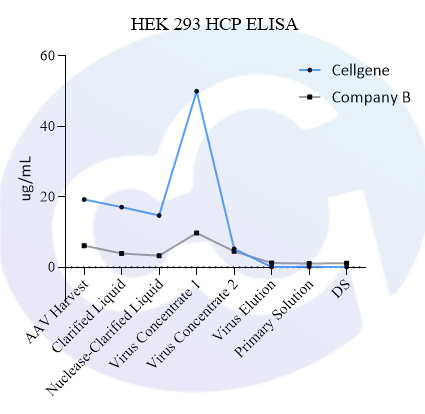
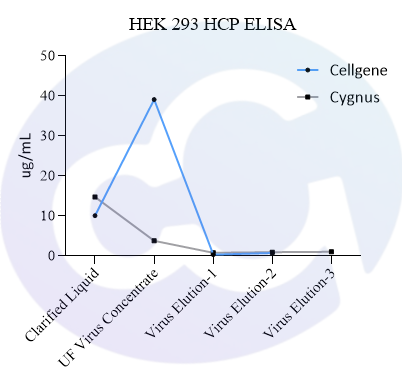
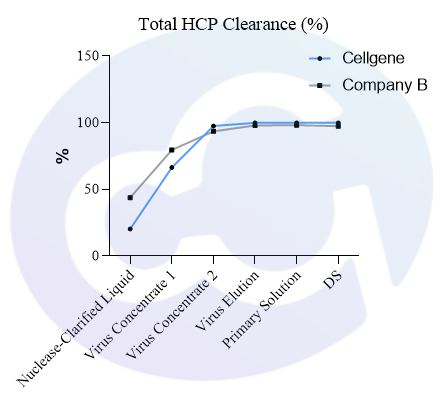
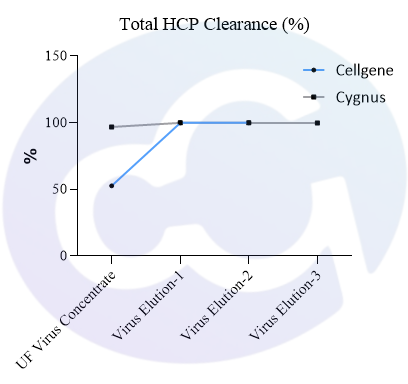
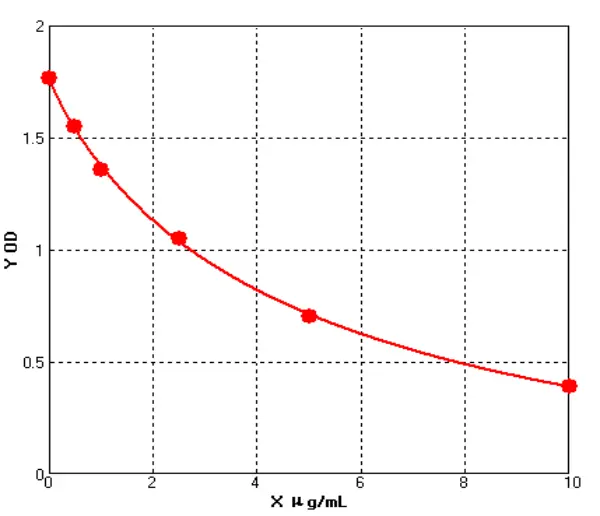
Meanwhile, a comparison was made with Cygnus' product (Catalogue No.: F650S) in terms of detection rate in different drugs (different purification stages of the same drug) of recombinant protein types, showing good comparability (see Figures 4 and 5).
Figures 4 |
Figures 5 |
In addition, in viral packaging samples (harvested liquid, clarified liquid, and viral concentrate), the detection results of this kit are mostly higher than those of Cygnus F650S and much higher than those of domestic parallel products. ELISA further verified the efficiency of customers' HCP clearance processes, which is consistent with the data accumulated from customers' years of purification processes. Moreover, due to the excellent recognition purity of HCP in the final product, it is easier to pass the approval of drug regulatory authorities.
Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
 Furthermore, Cellgene Bioscience has conducted sufficient specificity tests (e.g., nuclease, trypsin, BSA, HCP, etc.), greatly eliminating non-specific recognition (see Table 4 below).
Furthermore, Cellgene Bioscience has conducted sufficient specificity tests (e.g., nuclease, trypsin, BSA, HCP, etc.), greatly eliminating non-specific recognition (see Table 4 below).
Table 4
Samples | Con ng/mL | OD | ng/mL | Cross-reaction |
Nuclease | 1000 | 0.013 | 0 | 0.00% |
800 | 0.0117 | 0 | 0.00% | |
Trypsin | 250 | 0.0205 | 0 | 0.00% |
125 | 0.014 | 0 | 0.00% | |
Bovine Serum Albumin | 32 | 0.0127 | 0 | 0.00% |
CHO HCP | 200 | 0.0213 | 0 | 0.00% |
E.coli HCP | 100 | 0.0143 | 0 | 0.00% |
SF9 | 400 | 0.017 | 0 | 0.00% |
Hansenula polymorpha HCP | 800 | 0.0825 | 0 | 0.00% |
Pichia pastori X-33 HCP | 800 | 0.0195 | 0 | 0.00% |
Saccharomyces cerevisiae HCP | 800 | 0.072 | 0 | 0.00% |
Pichia pastori GS115 HCP | 100 | 0.016 | 0 | 0.00% |
Human IgM | 50 | 0.011 | 0 | 0.00% |
Human IgG | 5 | 0.0115 | 0 | 0.00% |
Cellgene Bioscience has always adhered to a problem-solving orientation and steadily advanced every step with pharmaceutical customers. "Authority" cannot replace technology to solve practical problems, and "gold standard" is not the only one. Instead, we should work with pharmaceutical companies to adhere to years of regulatory accumulation. Only by solving ELISA problems can we solve the subsequent LC-MS identification problems. Being disconnected from reality is dangerous, and the either-or approach is undesirable.
References:
[1] Kavsan, Vadym M; et al. Immortalized cells and one oncogene in malignant transformation: old insights on new explanation. BMC Cell Biology. 2011. 12: 23.
[2] Yao-Cheng Lin, et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nature communications. 2014. 5:4767.
[3] Louis N, et al. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997. 233 (2): 423-9.
Cellgene Bioscience has been dedicated to the biopharmaceutical and industrial testing field for 15 years, offering a series of HCP residual detection products, as well as comprehensive technical services including HCP-specific antibody development and coverage analysis.
Cellgene Bioscience-Drug Residue Detection Products | |
Host Cell Protein ELISA kits (HCP) | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G2 | |
CHO Host Cell Protein (CHO HCP) ELISA kit, G3 | |
HEK293 Host Cell Protein (HEK293 HCP) ELISA kit, G2 | |
E. coli Host Cell Protein (E. coli HCP) ELISA kit, G3 | |
Pichia pastoris Host Cell Protein (PP HCP) ELISA kit, G3 | |
Ogataea polymorpha Host Cell Protein ELISA kit, G3 | |
Saccharomyces cerevisiae Host Cell Protein ELISA kit, G3 | |
Spodoptera frugiperda (Sf9) Host Cell Protein ELISA kit, G3 | |
Medium Residues Detection kits | |
Protein A ELISA Kit (Boiling) | |
Protein A ELISA Kit | |
Mouse Immunoglobulin G ELISA Kit | |
Bovine Immunoglobulin G ELISA Kit | |
Human Immunoglobulin G ELISA Kit | |
Goat Immunoglobulin G ELISA Kit | |
Kanamycin ELISA Kit | |
Protein L ELISA Kit | |
NEGEP1271 | Protein G ELISA Kit |
Bovine Serum Albumin ELISA Kit | |
Human Serum Albumin ELISA Kit | |
Dextran Sulfate Salt Detection kit | |
Host Cell DNA Detection kits (HCD) | |
NS0 Host Cell DNA (NS0 HCD) Residue Detection kit | |
E.coli Host Cell DNA (E.coli HCD) Residue Detection kit | |
Vero Host Cell DNA (Vero HCD) Residue Detection kit | |
HEK293 Host Cell DNA (HEK293 HCD) Residue Detection kit | |
CHO Host Cell DNA (CHO HCD) Residue Detection Kit | |
Pichia Pastoris Host Cell DNA (PP HCD) Residue Detection Kit | |
Magnetic Residual DNA Sample Preparation Kit | |
Residual Total RNA Detection Kit | |
E.coli Residual Total RNA Detection Kit (qRT-PCR) | |
Host Cell Protein Antibodies | |
CHO Host Cell Protein G3 Antibody | |
CHO Host Cell Protein G2 Antibody | |
E.coli Host Cell Protein G3 Antibody | |
PH-E0021-2-Ab | Pichia Yeast Host Cell Protein G2 Antibody |
HEK293 Host Cell Protein G2 Antibody | |
Buffer Products | |
CG-H0100 | HCP ELISA buffer |
Protein L ELISA buffer | |