Search ELISA Kits
Bluegene Biotech/Cellgene Bioscience Host Cell Protein ELISA Kits Types
Reliable Quality, End-to-End Control
-
• With 20 years of deep technical expertise, we possess a professional and efficient R&D team with extensive problem-solving experience.
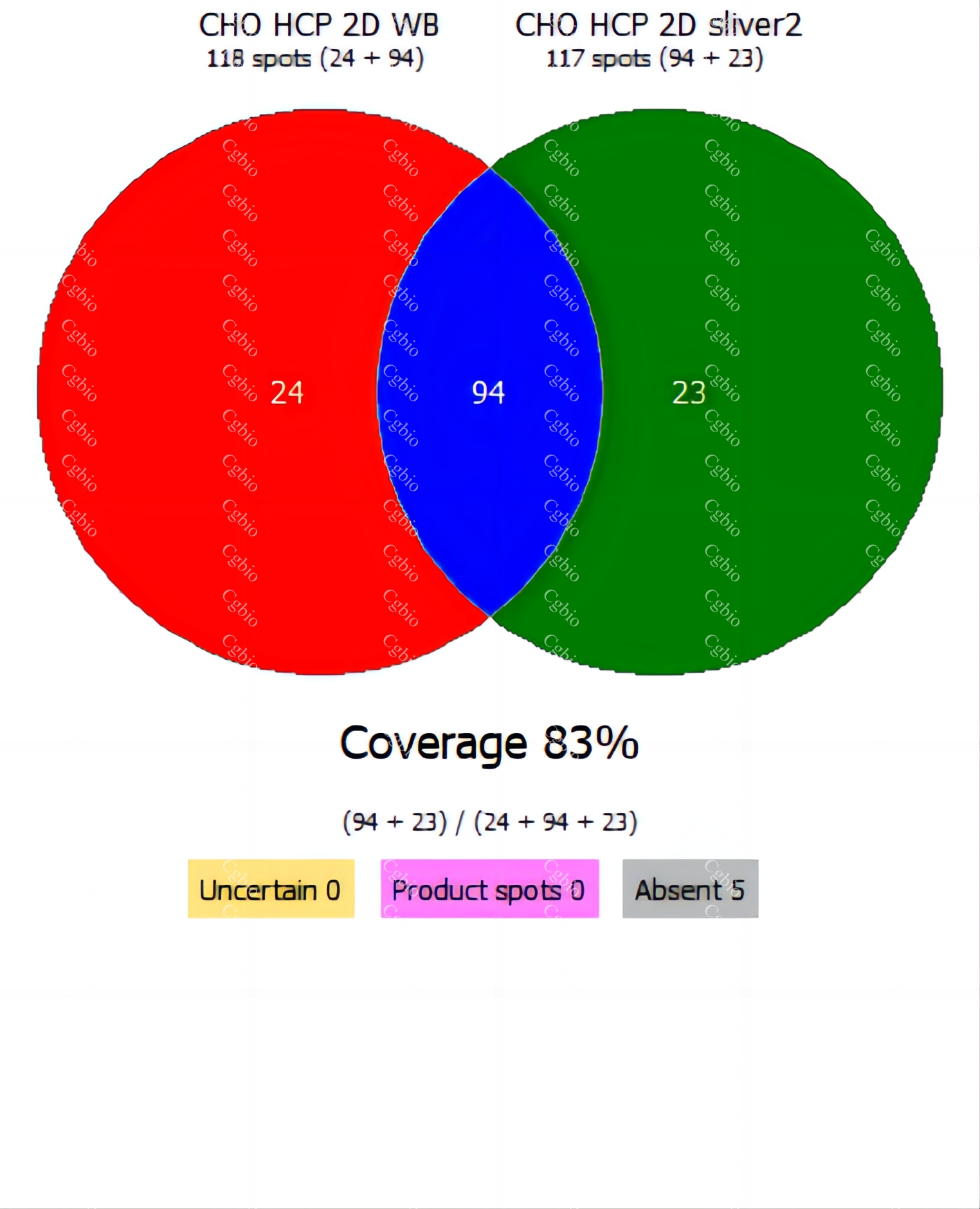
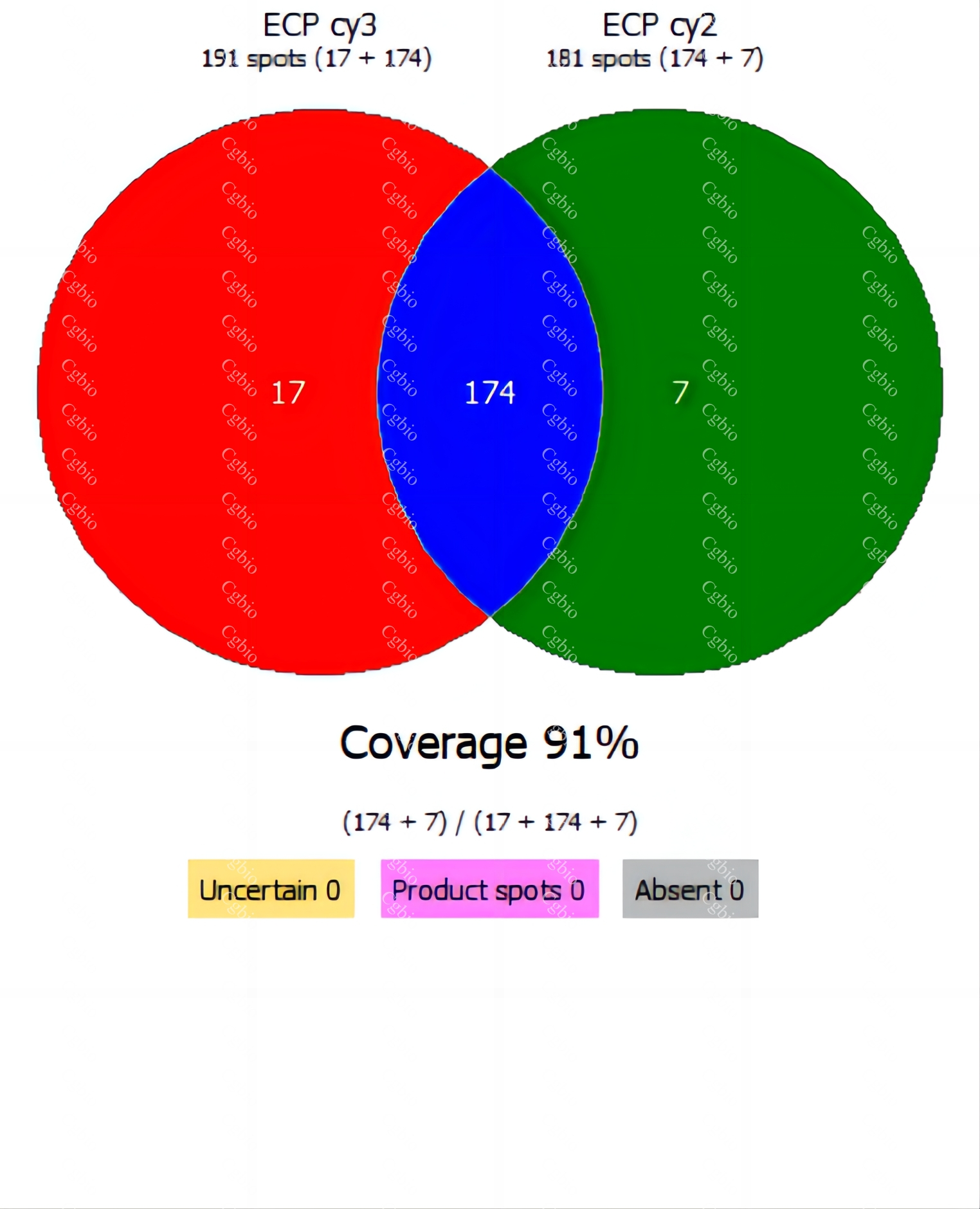
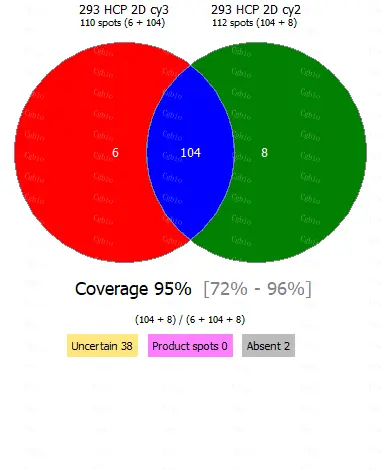
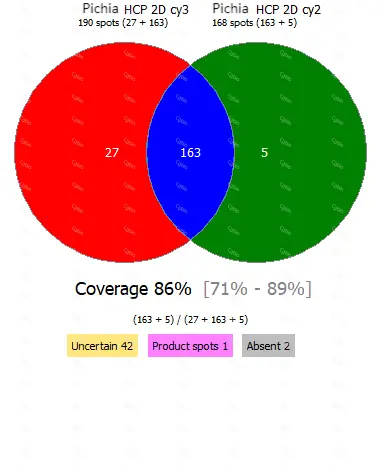
• Equipped with professional 2D-AAE and LC-MS combined coverage validation analysis software, capable of providing coverage detection for antibody raw materials from different batches.
• Establish a complete electronic archiving system for research and development data. All data are electronically retained in accordance with standards, and the complete set of data required for customer audits can be provided at any time. Ensure that the data results are true and reliable, with full traceability throughout the process, and fully meet the requirements of audit compliance.
• Supported by sufficient strategic reserves of key raw materials to eliminate batch-change risks at source, our highly streamlined production system has demonstrated extreme stability across numerous historical batches, providing robust assurance for quality and efficiency.
• From R&D to production, we maintain complete autonomy over the entire process—including core technologies, the manufacturing system, and quality control. This integrated self-sufficiency guarantees reliable supply stability.
• Establish an ISO13485:2016 medical device quality management system, covering the entire lifecycle management of product development, production, delivery, and after-sales.
Requirement of the Regulation (FDA, EMA, ICH)
-
FDA, EMA, and ICH all have precise regulations for the Host Cell Protein residues.
FDA requires that potential contaminants (including Host Cell Proteins ) introduced by the purification process should be below detectable levels using a highly sensitive method.

EMA also made a statement, Residual host cell DNA and residual host cell proteins are among the impurities to be eliminated, for HCP, whatever the product and production system, residual HCP has to be tested for on a routine basis.

ICH: For host cell proteins, a sensitive assay is utilized, and the removal of cell substrate-derived impurities such as HCP may sometimes be used to eliminate the need for establishing acceptance criteria for these impurities.

Examples of HCP Related Issues with Biologics
-
In 2006, an immunogenicity issue occurred to Omnitrope (rHGH biosimilar), the cause of which was linked to excess host cell protein contamination.
In 2008, Alpheon and Roferon-A finished products were also investigated for the composition of the different impurities that were not fully studied.
In 2013, IB1001 was a recombinant product for treatment and prevention, the clinical studies of which were on clinical hold due to a higher-than-expected rate of host cell antibody development in people treated with IB001.