E01B0391 Human Bone Morphogenetic Protein 9 ELISA kit
The Human Bone Morphogenetic Protein 9 ELISA kit can be used to identify samples from the human species. Bone Morphogenetic Protein 9 can also be called BMP9, GDF2, BMP-9, BMP9, HHT5, GDF-2, growth differentiation factor 2.



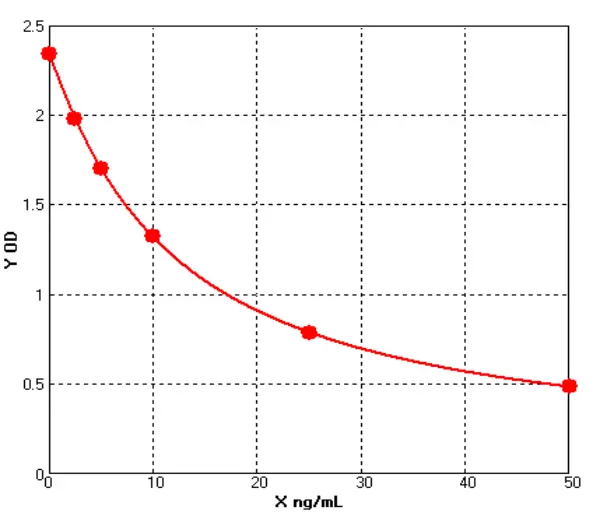
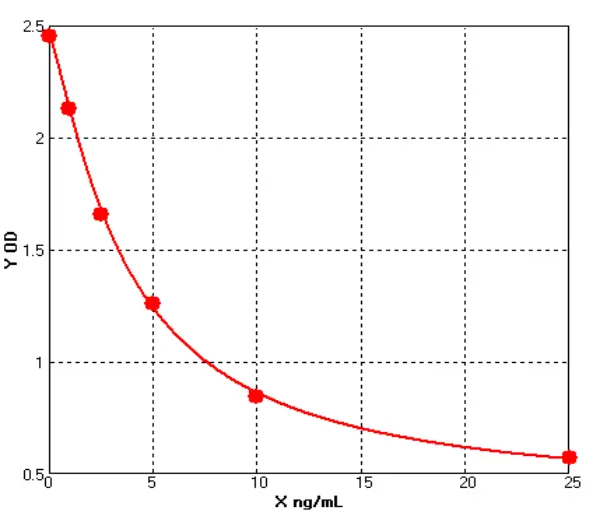
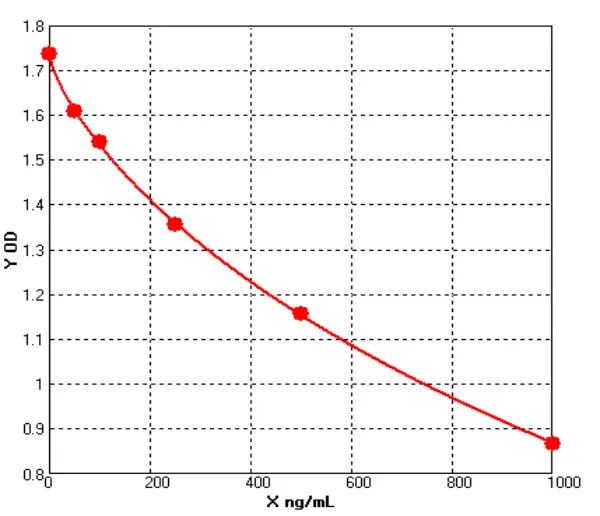
_00(1).webp)